Antimonene: a tuneable post-graphene material for advanced applications in optoelectronics, catalysis, energy and biomedicine
- PMID: 36744431
- PMCID: PMC9987414
- DOI: 10.1039/d2cs00570k
Antimonene: a tuneable post-graphene material for advanced applications in optoelectronics, catalysis, energy and biomedicine
Abstract
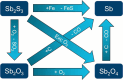
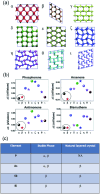
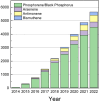
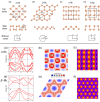
The post-graphene era is undoubtedly marked by two-dimensional (2D) materials such as quasi-van der Waals antimonene. This emerging material has a fascinating structure, exhibits a pronounced chemical reactivity (in contrast to graphene), possesses outstanding electronic properties and has been postulated for a plethora of applications. However, chemistry and physics of antimonene remain in their infancy, but fortunately recent discoveries have shed light on its unmatched allotropy and rich chemical reactivity offering a myriad of unprecedented possibilities in terms of fundamental studies and applications. Indeed, antimonene can be considered as one of the most appealing post-graphene 2D materials reported to date, since its structure, properties and applications can be chemically engineered from the ground up (both using top-down and bottom-up approaches), offering an unprecedented level of control in the realm of 2D materials. In this review, we provide an in-depth analysis of the recent advances in the synthesis, characterization and applications of antimonene. First, we start with a general introduction to antimonene, and then we focus on its general chemistry, physical properties, characterization and synthetic strategies. We then perform a comprehensive study on the allotropy, the phase transition mechanisms, the oxidation behaviour and chemical functionalization. From a technological point of view, we further discuss the applications recently reported for antimonene in the fields of optoelectronics, catalysis, energy storage, cancer therapy and sensing. Finally, important aspects such as new scalable methodologies or the promising perspectives in biomedicine are discussed, pinpointing antimonene as a cutting-edge material of broad interest for researchers working in chemistry, physics, materials science and biomedicine.
Conflict of interest statement
There are no conflicts to declare.
Figures




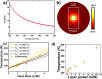

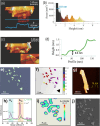


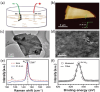





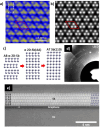
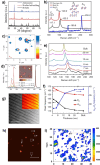

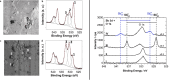








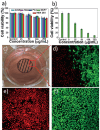
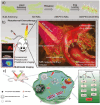




References
-
- Butler S. Z. Hollen S. M. Cao L. Cui Y. Gupta J. A. Gutiérrez H. R. Heinz T. F. Hong S. S. Huang J. Ismach A. F. Johnston-Halperin E. Kuno M. Plashnitsa V. V. Robinson R. D. Ruoff R. S. Salahuddin S. Shan J. Shi L. Spencer M. G. Terrones M. Windl W. Goldberger J. E. Progress, Challenges, and Opportunities in Two-Dimensional Materials Beyond Graphene. ACS Nano. 2013;7(4):2898–2926. doi: 10.1021/nn400280c. doi: 10.1021/nn400280c. - DOI - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

