Absorption rate governs cell transduction in dry macroporous scaffolds
- PMID: 36744434
- PMCID: PMC10050106
- DOI: 10.1039/d2bm01753a
Absorption rate governs cell transduction in dry macroporous scaffolds
Abstract
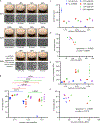
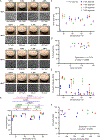
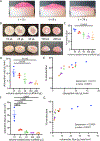
Developing the next generation of cellular therapies will depend on fast, versatile, and efficient cellular reprogramming. Novel biomaterials will play a central role in this process by providing scaffolding and bioactive signals that shape cell fate and function. Previously, our lab reported that dry macroporous alginate scaffolds mediate retroviral transduction of primary T cells with efficiencies that rival the gold-standard clinical spinoculation procedures, which involve centrifugation on Retronectin-coated plates. This scaffold transduction required the scaffolds to be both macroporous and dry. Transduction by dry, macroporous scaffolds, termed "Drydux transduction," provides a fast and inexpensive method for transducing cells for cellular therapy, including for the production of CAR T cells. In this study, we investigate the mechanism of action by which Drydux transduction works through exploring the impact of pore size, stiffness, viral concentration, and absorption speed on transduction efficiency. We report that Drydux scaffolds with macropores ranging from 50-230 μm and with Young's moduli ranging from 25-620 kPa all effectively transduce primary T cells, suggesting that these parameters are not central to the mechanism of action, but also demonstrating that Drydux scaffolds can be tuned without losing functionality. Increasing viral concentrations led to significantly higher transduction efficiencies, demonstrating that increased cell-virus interaction is necessary for optimal transduction. Finally, we discovered that the rate with which the cell-virus solution is absorbed into the scaffold is closely correlated to viral transduction efficiency, with faster absorption producing significantly higher transduction. A computational model of liquid flow through porous media validates this finding by showing that increased fluid flow substantially increases collisions between virus particles and cells in a porous scaffold. Taken together, we conclude that the rate of liquid flow through the scaffolds, rather than pore size or stiffness, serves as a central regulator for efficient Drydux transduction.
Conflict of interest statement
Conflicts of interest
Y.B. is an inventor on patents related to the use of biomaterials for generation of CAR-T cell therapeutics and receives an industry-sponsored research grant related to CAR-T cell therapeutic technology (unrelated to this work). All other authors declare that they have no competing interests.
Figures
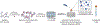
References
-
- Kim JK; Kim HJ; Chung J-Y; Lee J-H; Young S-B; Kim Y-H Natural and Synthetic Biomaterials for Controlled Drug Delivery. Arch. Pharm. Res. 2014, 37 (1), 60–68. - PubMed
-
- Hamid Akash MS; Rehman K; Chen S Natural and Synthetic Polymers as Drug Carriers for Delivery of Therapeutic Proteins. Polym. Rev. 2015, 55 (3), 371–406.
-
- Soni SS; Rodell CB Polymeric Materials for Immune Engineering: Molecular Interaction to Biomaterial Design. Acta Biomater. 2021, 133, 139–152. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

