Mechanism of strand displacement DNA synthesis by the coordinated activities of human mitochondrial DNA polymerase and SSB
- PMID: 36744436
- PMCID: PMC9976888
- DOI: 10.1093/nar/gkad037
Mechanism of strand displacement DNA synthesis by the coordinated activities of human mitochondrial DNA polymerase and SSB
Abstract
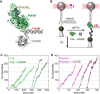
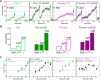
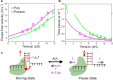
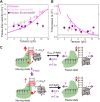
Many replicative DNA polymerases couple DNA replication and unwinding activities to perform strand displacement DNA synthesis, a critical ability for DNA metabolism. Strand displacement is tightly regulated by partner proteins, such as single-stranded DNA (ssDNA) binding proteins (SSBs) by a poorly understood mechanism. Here, we use single-molecule optical tweezers and biochemical assays to elucidate the molecular mechanism of strand displacement DNA synthesis by the human mitochondrial DNA polymerase, Polγ, and its modulation by cognate and noncognate SSBs. We show that Polγ exhibits a robust DNA unwinding mechanism, which entails lowering the energy barrier for unwinding of the first base pair of the DNA fork junction, by ∼55%. However, the polymerase cannot prevent the reannealing of the parental strands efficiently, which limits by ∼30-fold its strand displacement activity. We demonstrate that SSBs stimulate the Polγ strand displacement activity through several mechanisms. SSB binding energy to ssDNA additionally increases the destabilization energy at the DNA junction, by ∼25%. Furthermore, SSB interactions with the displaced ssDNA reduce the DNA fork reannealing pressure on Polγ, in turn promoting the productive polymerization state by ∼3-fold. These stimulatory effects are enhanced by species-specific functional interactions and have significant implications in the replication of the human mitochondrial DNA.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

References
-
- Kaguni L.S. DNA polymerase gamma, the mitochondrial replicase. Annu. Rev. Biochem. 2004; 73:293–320. - PubMed
-
- Lee H.R., Johnson K.A.. Fidelity of the human mitochondrial DNA polymerase. J. Biol. Chem. 2006; 281:36236–36240. - PubMed
-
- Johnson A.A., Johnson K.A.. Fidelity of nucleotide incorporation by human mitochondrial DNA polymerase. J. Biol. Chem. 2001; 276:38090–38096. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

