Comparing Variability in Measurement of Subcutaneous Tumors in Mice Using 3D Thermal Imaging and Calipers
- PMID: 36744510
- PMCID: PMC9827613
- DOI: 10.30802/AALAS-CM-22-000033
Comparing Variability in Measurement of Subcutaneous Tumors in Mice Using 3D Thermal Imaging and Calipers
Abstract
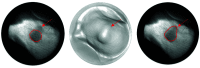
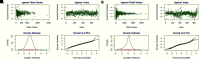
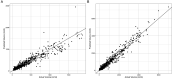
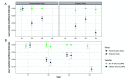
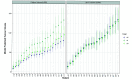
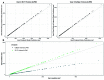
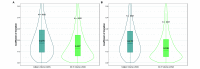
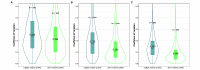
Repeatable tumor measurements are key to accurately assessing tumor growth and treatment efficacy. A preliminary study that we conducted showed that a novel 3D and thermal imaging system (3D-TI) for measuring subcutaneous tumors in rodents significantly reduced interoperator variability across 3 in vivo efficacy studies. Here we further studied this reduction in interoperator variability across a much larger dataset. A dataset consisting of 6,532 paired 3D-TI and caliper interoperator measurements was obtained from tumor scans and measurements in 27 laboratories across 289 studies, 153 operators, over 20 mouse strains, and 100 cell lines. Interoperator variability in both measurement methods was analyzed using coefficient of variation (CV), intraclass correlation (ICC) analysis, and significance testing. The median 3D-TI CV was significantly lower than the median caliper CV. The effects of large interoperator variability at critical points in the study were also investigated. At stratified randomization, changing the operator performing caliper measurements resulted in a 59% probability that a mouse would be reassigned to a different group. The probability that this would occur when using 3D-TI was significantly lower at 29%. In studies in which a tumor was expected to regress, changing the operator during the study was associated with a tumor volume increase of approximately 500mm³ when using calipers. This change did not occur when using 3D-TI. We conclude that 3D-TI significantly reduces interoperator variability as compared with calipers and can improve reproducibility of in vivo studies across a wide range of mouse strains and cell lines.
Figures




Comment in
-
Letter to the Editor.Comp Med. 2023 Apr 1;73(2):107. Comp Med. 2023. PMID: 37170457 Free PMC article. No abstract available.
References
-
- Delgado-SanMartin J, Ehrhardt B, Paczkowski M, Hackett S, Smith A, Waraich W, Klatzow J, Zabair A, Chabokdast A, Rubio-Navarro L, Rahi A, Wilson Z. 2019. An innovative non-invasive technique for subcutaneous tumour measurements. PLoS One 14:e0216690. 10.1371/journal.pone.0216690. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

