Eliminating Potential Effects of Other Infections During Selection of Nonhuman Primates for COVID-19 Research
- PMID: 36744555
- PMCID: PMC9948906
- DOI: 10.30802/AALAS-CM-21-000086
Eliminating Potential Effects of Other Infections During Selection of Nonhuman Primates for COVID-19 Research
Abstract
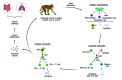
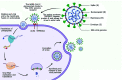
The study of nonhuman primates (NHP) can provide significant insights into our understanding numerous infectious agents. The etiological agent of COVID-19, SARS-CoV-2 virus, first emerged in 2019 and has so far been responsible for the deaths of over 4 million people globally. In the frenzied search to understand its pathogenesis and immunology and to find measures for prevention and control of this pandemic disease, NHP, particularly macaques, are the preferred model because they manifest similar clinical signs and immunologic features as humans. However, possible latent, subclinical, and opportunistic infections not previously detected in animals participating in a study may obscure experimental results and confound data interpretations in testing treatments and vaccine studies for COVID-19. Certain pathophysiologic changes that occur with SARS-CoV-2 virus infection are similar to those of simian pathogens. The current review discusses numerous coinfections of COVID-19 with other diseases and describes possible outcomes and mechanisms in COVID-19 studies of NHP that have coinfections. Due to the urgency triggered by the pandemic, screening that is more rigorous than usual is necessary to limit background noise and maximize the reliability of data from NHP COVID-19 studies. Screening for influenza virus, selected respiratory bacteria, and regional endemic pathogens such as vector-borne agents, together with the animal's individual exposure history, should be the main considerations in selecting a NHP for a COVID-19 study. In addition, because NHP are susceptible to the SARS-CoV-2 virus, management and surveillance measures should be established to prevent transmission to healthy animals from infected colony animals and husbandry staff. This review presents compiled data on the use of NHP in COVID-19 studies, emphasizing the need to create the most reliable NHP model for those studies by extensive screening for other pathogens.
Figures
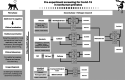

References
-
- Andrade A, Andrade MCR, Marinho AM, Ferreira-Filho J. 2010. Biologia, manejo e medicina de primatas não humanos na pesquisa biomédica. [Biology, management and medicine of non-human primates in biomedical research]. Rio de Janeiro: Fiocruz. 472 p.
-
- Andrade MCR. 2017. Primatas não humanos para estudos biomédicos: Manejo, agentes infecciosos e monitoramento sanitário. [Non-human primates for biomedical studies: management, infectious agents, and health monitoring] Rev Soc Bras Ci Anim Lab 5:64–75 [Article in Portugese].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

