Surface or Internal Hydration - Does It Really Matter?
- PMID: 36744598
- PMCID: PMC9983018
- DOI: 10.1021/jasms.2c00290
Surface or Internal Hydration - Does It Really Matter?
Abstract
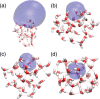
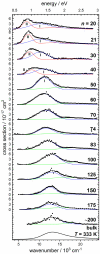
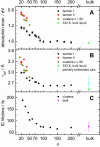
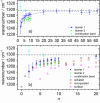
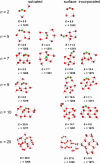
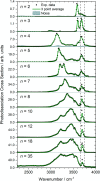
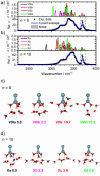
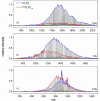
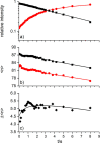
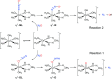
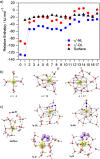
The precise location of an ion or electron, whether it is internally solvated or residing on the surface of a water cluster, remains an intriguing question. Subtle differences in the hydrogen bonding network may lead to a preference for one or the other. Here we discuss spectroscopic probes of the structure of gas-phase hydrated ions in combination with quantum chemistry, as well as H/D exchange as a means of structure elucidation. With the help of nanocalorimetry, we look for thermochemical signatures of surface vs internal solvation. Examples of strongly size-dependent reactivity are reviewed which illustrate the influence of surface vs internal solvation on unimolecular rearrangements of the cluster, as well as on the rate and product distribution of ion-molecule reactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

