Application of Magnetocardiography to Screen for Inflammatory Cardiomyopathy and Monitor Treatment Response
- PMID: 36744683
- PMCID: PMC10111485
- DOI: 10.1161/JAHA.122.027619
Application of Magnetocardiography to Screen for Inflammatory Cardiomyopathy and Monitor Treatment Response
Abstract
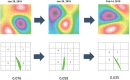
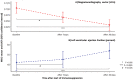
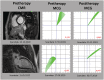
Background Inflammatory cardiomyopathy is one of the most common causes of sudden cardiac death in young adults. Diagnosis of inflammatory cardiomyopathy remains challenging, and better monitoring tools are needed. We present magnetocardiography as a method to diagnose myocardial inflammation and monitor treatment response. Methods and Results A total of 233 patients were enrolled, with a mean age of 45 (±18) years, and 105 (45%) were women. The primary analysis included 209 adult subjects, of whom 66 (32%) were diagnosed with inflammatory cardiomyopathy, 17 (8%) were diagnosed with cardiac amyloidosis, and 35 (17%) were diagnosed with other types of nonischemic cardiomyopathy; 91 (44%) did not have cardiomyopathy. The second analysis included 13 patients with inflammatory cardiomyopathy who underwent immunosuppressive therapy after baseline magnetocardiography measurement. Finally, diagnostic accuracy of magnetocardiography was tested in 3 independent cohorts (total n=23) and 1 patient, who developed vaccine-related myocarditis. First, we identified a magnetocardiography vector to differentiate between patients with cardiomyopathy versus patients without cardiomyopathy (vector of ≥0.051; sensitivity, 0.59; specificity, 0.95; positive predictive value, 93%; and negative predictive value, 64%). All patients with inflammatory cardiomyopathy, including a patient with mRNA vaccine-related myocarditis, had a magnetocardiography vector ≥0.051. Second, we evaluated the ability of the magnetocardiography vector to reflect treatment response. We observed a decrease of the pathologic magnetocardiography vector toward normal in all 13 patients who were clinically improving under immunosuppressive therapy. Magnetocardiography detected treatment response as early as day 7, whereas echocardiographic detection of treatment response occurred after 1 month. The magnetocardiography vector decreased from 0.10 at baseline to 0.07 within 7 days (P=0.010) and to 0.03 within 30 days (P<0.001). After 30 days, left ventricular ejection fraction improved from 42.2% at baseline to 53.8% (P<0.001). Conclusions Magnetocardiography has the potential to be used for diagnostic screening and to monitor early treatment response. The method is valuable in inflammatory cardiomyopathy, where there is a major unmet need for early diagnosis and monitoring response to immunosuppressive therapy.
Keywords: COVID‐19; echocardiography; ejection fraction; immunosuppressive therapy; inflammatory cardiomyopathy; magnetocardiography.
Figures



References
-
- Heidecker BCL. Acute Fulminant Myocarditis. In: Brown DL, ed. Cardiac Intensive Care. 3rd ed. Elsevier; 2019:199–204.
-
- Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. 2648a‐2648d. doi: 10.1093/eurheartj/eht210 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

