Mouse guanylate-binding protein 1 does not mediate antiviral activity against influenza virus in vitro or in vivo
- PMID: 36744765
- PMCID: PMC10952839
- DOI: 10.1111/imcb.12627
Mouse guanylate-binding protein 1 does not mediate antiviral activity against influenza virus in vitro or in vivo
Abstract
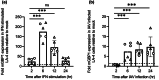
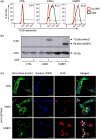
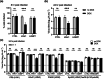
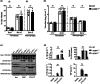
Many interferon (IFN)-stimulated genes are upregulated within host cells following infection with influenza and other viruses. While the antiviral activity of some IFN-stimulated genes, such as the IFN-inducible GTPase myxoma resistance (Mx)1 protein 1, has been well defined, less is known regarding the antiviral activities of related IFN-inducible GTPases of the guanylate-binding protein (GBP) family, particularly mouse GBPs, where mouse models can be used to assess their antiviral properties in vivo. Herein, we demonstrate that mouse GBP1 (mGBP1) was upregulated in a mouse airway epithelial cell line (LA-4 cells) following pretreatment with mouse IFNα or infection by influenza A virus (IAV). Whereas doxycycline-inducible expression of mouse Mx1 (mMx1) in LA-4 cells resulted in reduced susceptibility to IAV infection and reduced viral growth, inducible mGBP1 did not. Moreover, primary cells isolated from mGBP1-deficient mice (mGBP1-/- ) showed no difference in susceptibility to IAV and mGBP1-/- macrophages showed no defect in IAV-induced NLRP3 (NLR family pyrin domain containing 3) inflammasome activation. After intranasal IAV infection, mGBP1-/- mice also showed no differences in virus replication or induction of inflammatory responses in the airways during infection. Thus, using complementary approaches such as mGBP1 overexpression, cells from mGBP1-/- mice and intranasal infection of mGBP1-/- we demonstrate that mGBP1 does not play a major role in modulating IAV infection in vitro or in vivo.
Keywords: GTPase; influenza A virus; innate immunity; interferon-stimulated gene.
© 2023 The Authors. Immunology & Cell Biology published by John Wiley & Sons Australia, Ltd on behalf of the Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Research Materials

