Covalent Tethers for Precise Amino Alcohol Syntheses: Ring Opening of Epoxides by Pendant Sulfamates and Sulfamides
- PMID: 36744823
- PMCID: PMC10017054
- DOI: 10.1021/acs.orglett.3c00053
Covalent Tethers for Precise Amino Alcohol Syntheses: Ring Opening of Epoxides by Pendant Sulfamates and Sulfamides
Abstract
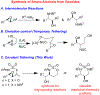
We describe the development of the first ring opening of epoxides using pendant sulfamates and sulfamides. These reactions are promoted by a base and proceed under mild conditions to afford oxathiazinanes and cyclic sulfamides with excellent diastereoselectivity and regiocontrol. The reactions scale well, and the products serve as synthons for ring-opening reactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Baran PS Natural Product Total Synthesis: As Exciting as Ever and Here To Stay. J. Am. Chem. Soc 2018, 140, 4751–4755. - PubMed
-
- Moschona F; Savvopoulou I; Tsitopoulou M; Tataraki D; Rassias G Epoxide Syntheses and Ring-Opening Reactions in Drug Development. Catalysts 2020, 10, 1117.
-
- Jacobsen EN Asymmetric Catalysis of Epoxide Ring-Opening Reactions. Acc. Chem. Res 2000, 33, 421–431. - PubMed
-
- Hanson RM The synthetic methodology of nonracemic glycidol and related 2,3-epoxy alcohols. Chem. Rev 1991, 91, 437–475.
Grants and funding
LinkOut - more resources
Full Text Sources

