Glycogen-Degrading Activities of Catalytic Domains of α-Amylase and α-Amylase-Pullulanase Enzymes Conserved in Gardnerella spp. from the Vaginal Microbiome
- PMID: 36744900
- PMCID: PMC9945562
- DOI: 10.1128/jb.00393-22
Glycogen-Degrading Activities of Catalytic Domains of α-Amylase and α-Amylase-Pullulanase Enzymes Conserved in Gardnerella spp. from the Vaginal Microbiome
Abstract
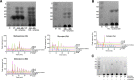
Gardnerella spp. are associated with bacterial vaginosis in which normally dominant lactobacilli are replaced with facultative and anaerobic bacteria, including Gardnerella spp. Co-occurrence of multiple species of Gardnerella is common in the vagina, and competition for nutrients such as glycogen likely contributes to the differential abundances of Gardnerella spp. Glycogen must be digested into smaller components for uptake, a process that depends on the combined action of glycogen-degrading enzymes. In this study, the ability of culture supernatants of 15 isolates of Gardnerella spp. to produce glucose, maltose, maltotriose, and maltotetraose from glycogen was demonstrated. Carbohydrate-active enzymes (CAZymes) were identified bioinformatically in Gardnerella proteomes using dbCAN2. Identified proteins included a single-domain α-amylase (EC 3.2.1.1) (encoded by all 15 isolates) and an α-amylase-pullulanase (EC 3.2.1.41) containing amylase, carbohydrate binding modules, and pullulanase domains (14/15 isolates). To verify the sequence-based functional predictions, the amylase and pullulanase domains of the α-amylase-pullulanase and the single-domain α-amylase were each produced in Escherichia coli. The α-amylase domain from the α-amylase-pullulanase released maltose, maltotriose, and maltotetraose from glycogen, and the pullulanase domain released maltotriose from pullulan and maltose from glycogen, demonstrating that the Gardnerella α-amylase-pullulanase is capable of hydrolyzing α-1,4 and α-1,6 glycosidic bonds. Similarly, the single-domain α-amylase protein also produced maltose, maltotriose, and maltotetraose from glycogen. Our findings show that Gardnerella spp. produce extracellular amylase enzymes as "public goods" that can digest glycogen into maltose, maltotriose, and maltotetraose that can be used by the vaginal microbiota. IMPORTANCE Increased abundance of Gardnerella spp. is a diagnostic characteristic of bacterial vaginosis, an imbalance in the human vaginal microbiome associated with troubling symptoms, and negative reproductive health outcomes, including increased transmission of sexually transmitted infections and preterm birth. Competition for nutrients is likely an important factor in causing dramatic shifts in the vaginal microbial community, but little is known about the contribution of bacterial enzymes to the metabolism of glycogen, a major food source available to vaginal bacteria. The significance of our research is characterizing the activity of enzymes conserved in Gardnerella species that contribute to the ability of these bacteria to utilize glycogen.
Keywords: Gardnerella; amylase; glycogen; human microbiome; pullulanase; vagina; vaginosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Characterization of an α-Glucosidase Enzyme Conserved in Gardnerella spp. Isolated from the Human Vaginal Microbiome.J Bacteriol. 2021 Aug 9;203(17):e0021321. doi: 10.1128/JB.00213-21. Epub 2021 Aug 9. J Bacteriol. 2021. PMID: 34124938 Free PMC article.
-
Transport and Utilization of Glycogen Breakdown Products by Gardnerella spp. from the Human Vaginal Microbiome.Microbiol Spectr. 2023 Mar 15;11(2):e0443522. doi: 10.1128/spectrum.04435-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36920187 Free PMC article.
-
Amylases in the Human Vagina.mSphere. 2020 Dec 9;5(6):e00943-20. doi: 10.1128/mSphere.00943-20. mSphere. 2020. PMID: 33298571 Free PMC article.
-
Cholesterol-Dependent Cytolysins Produced by Vaginal Bacteria: Certainties and Controversies.Front Cell Infect Microbiol. 2020 Jan 10;9:452. doi: 10.3389/fcimb.2019.00452. eCollection 2019. Front Cell Infect Microbiol. 2020. PMID: 31998661 Free PMC article. Review.
-
Gardnerella and vaginal health: the truth is out there.FEMS Microbiol Rev. 2020 Jan 1;44(1):73-105. doi: 10.1093/femsre/fuz027. FEMS Microbiol Rev. 2020. PMID: 31697363 Review.
Cited by
-
Inferred bi-directional interactions between vaginal microbiota, metabolome and persistent HPV infection accompanied by high-grade cervical intraepithelial neoplasia.BMC Microbiol. 2025 Jul 2;25(1):404. doi: 10.1186/s12866-025-04126-w. BMC Microbiol. 2025. PMID: 40604390 Free PMC article.
-
Transformation of Gardnerella vaginalis with a Bifidobacterium-Escherichia coli shuttle vector plasmid.Microbiol Spectr. 2025 May 6;13(5):e0048125. doi: 10.1128/spectrum.00481-25. Epub 2025 Apr 10. Microbiol Spectr. 2025. PMID: 40207948 Free PMC article.
-
Syntrophic bacterial and host-microbe interactions in bacterial vaginosis.ISME J. 2025 Jan 2;19(1):wraf055. doi: 10.1093/ismejo/wraf055. ISME J. 2025. PMID: 40576334 Free PMC article.
-
Degradation of Resistant α-1,4-glucan by Vaginal Gardnerella Species is Associated with Bacterial Vaginosis.Curr Microbiol. 2025 Sep 2;82(10):487. doi: 10.1007/s00284-025-04459-9. Curr Microbiol. 2025. PMID: 40892095 Free PMC article.
-
Bacterial amylases enable glycogen degradation by the vaginal microbiome.Nat Microbiol. 2023 Sep;8(9):1641-1652. doi: 10.1038/s41564-023-01447-2. Epub 2023 Aug 10. Nat Microbiol. 2023. PMID: 37563289 Free PMC article.
References
-
- Vaneechoutte M, Guschin A, Van Simaey L, Gansemans Y, Van Nieuwerburgh F, Cools P. 2019. Emended description of Gardnerella vaginalis and description of Gardnerella leopoldii sp. nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp. nov., with delineation of 13 genomic species within the genus Gardnerella. Int J Syst Evol Microbiol 69:679–687. 10.1099/ijsem.0.003200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

