Comprehensive analysis of germline drivers in endometrial cancer
- PMID: 36744932
- PMCID: PMC10165491
- DOI: 10.1093/jnci/djad016
Comprehensive analysis of germline drivers in endometrial cancer
Abstract
Background: We sought to determine the prevalence of germline pathogenic variants (gPVs) in unselected patients with endometrial cancer (EC), define biallelic gPVs within tumors, and describe their associations with clinicopathologic features.
Methods: Germline assessment of at least 76 cancer predisposition genes was performed in patients with EC undergoing clinical tumor-normal Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) sequencing from January 1, 2015, to June 30, 2021. In patients with gPVs, biallelic alterations in ECs were identified through analysis of loss of heterozygosity and somatic PVs. Clinicopathologic variables were compared using nonparametric tests.
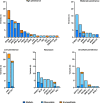
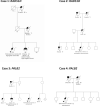
Results: Of 1625 patients with EC, 216 (13%) had gPVs, and 15 patients had 2 gPVs. There were 231 gPVs in 35 genes (75 [32%] high penetrance; 39 [17%] moderate penetrance; and 117 [51%] low, recessive, or uncertain penetrance). Compared with those without gPVs, patients with gPVs were younger (P = .002), more often White (P = .009), and less obese (P = .025) and had differences in distribution of tumor histology (P = .017) and molecular subtype (P < .001). Among 231 gPVs, 74 (32%) exhibited biallelic inactivation within tumors. For high-penetrance gPVs, 63% (47 of 75) of ECs had biallelic alterations, primarily affecting mismatch repair (MMR) and homologous recombination related genes, including BRCA1,BRCA2, RAD51D, and PALB2. Biallelic inactivation varied across molecular subtypes with highest rates in microsatellite instability-high (MSI-H) or copy-number (CN)-high subtypes (3 of 12 [25%] POLE, 30 of 77 [39%] MSI-H, 27 of 60 [45%] CN-high, 9 of 57 [16%] CN-low; P < .001).
Conclusions: Of unselected patients with EC, 13% had gPVs, with 63% of gPVs in high-penetrance genes (MMR and homologous recombination) exhibiting biallelic inactivation, potentially driving cancer development. This supports germline assessment in EC given implications for treatment and cancer prevention.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Kahn RM, Gordhandas S, Maddy BP, et al. Universal endometrial cancer tumor typing: how much has immunohistochemistry, microsatellite instability, and MLH1 methylation improved the diagnosis of Lynch syndrome across the population? Cancer. 2019;125(18):3172-3183. - PubMed
-
- Talhouk A, McConechy MK, Leung S, et al. Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer. 2017;123(5):802-813. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

