Geographic variation in bacterial assemblages on cane toad skin is influenced more by local environments than by evolved changes in host traits
- PMID: 36745034
- PMCID: PMC9932784
- DOI: 10.1242/bio.059641
Geographic variation in bacterial assemblages on cane toad skin is influenced more by local environments than by evolved changes in host traits
Abstract
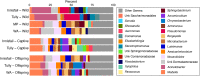
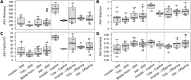
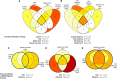
Bacterial assemblages on amphibian skin may play an important role in protecting hosts against infection. In hosts that occur over a range of environments, geographic variation in composition of bacterial assemblages might be due to direct effects of local factors and/or to evolved characteristics of the host. Invasive cane toads (Rhinella marina) are an ideal candidate to evaluate environmental and genetic mechanisms, because toads have evolved major shifts in physiology, morphology, and behavior during their brief history in Australia. We used samples from free-ranging toads to quantify site-level differences in bacterial assemblages and a common-garden experiment to see if those differences disappeared when toads were raised under standardised conditions at one site. The large differences in bacterial communities on toads from different regions were not seen in offspring raised in a common environment. Relaxing bacterial clustering to operational taxonomic units in place of amplicon sequence variants likewise revealed high similarity among bacterial assemblages on toads in the common-garden study, and with free-ranging toads captured nearby. Thus, the marked geographic divergence in bacterial assemblages on wild-caught cane toads across their Australian invasion appears to result primarily from local environmental effects rather than evolved shifts in the host.
Keywords: Bufo marinus; Invasive species; Rapid evolution; Skin bacteria.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

Similar articles
-
Differential Temporal Shifts in Skin Bacteria on Wild and Captive Toads.Microb Ecol. 2025 Apr 29;88(1):35. doi: 10.1007/s00248-025-02537-w. Microb Ecol. 2025. PMID: 40301143 Free PMC article.
-
The accelerating anuran: evolution of locomotor performance in cane toads (Rhinella marina, Bufonidae) at an invasion front.Proc Biol Sci. 2020 Nov 11;287(1938):20201964. doi: 10.1098/rspb.2020.1964. Epub 2020 Nov 11. Proc Biol Sci. 2020. PMID: 33171090 Free PMC article.
-
Brain transcriptome analysis reveals gene expression differences associated with dispersal behaviour between range-front and range-core populations of invasive cane toads in Australia.Mol Ecol. 2022 Mar;31(6):1700-1715. doi: 10.1111/mec.16347. Epub 2022 Jan 28. Mol Ecol. 2022. PMID: 35028988 Free PMC article.
-
The ecological impact of invasive cane toads (Bufo marinus) in Australia.Q Rev Biol. 2010 Sep;85(3):253-91. doi: 10.1086/655116. Q Rev Biol. 2010. PMID: 20919631 Review.
-
A genetic perspective on rapid evolution in cane toads (Rhinella marina).Mol Ecol. 2015 May;24(9):2264-76. doi: 10.1111/mec.13184. Epub 2015 Apr 20. Mol Ecol. 2015. PMID: 25894012 Review.
Cited by
-
From Skin to Gut: Understanding Microbial Diversity in Rana amurensis and R. dybowskii.Curr Microbiol. 2024 Sep 13;81(11):354. doi: 10.1007/s00284-024-03868-6. Curr Microbiol. 2024. PMID: 39269482
-
Differential Temporal Shifts in Skin Bacteria on Wild and Captive Toads.Microb Ecol. 2025 Apr 29;88(1):35. doi: 10.1007/s00248-025-02537-w. Microb Ecol. 2025. PMID: 40301143 Free PMC article.
References
-
- Abarca, J. G., Zuniga, I., Ortiz-Morales, G., Lugo, A., Viquez-Cervilla, M., Rodriguez-Hernandez, N., Vázquez-Sánchez, F., Murillo-Cruz, C., Torres-Rivera, E. A., Pinto-Tomás, A. A.et al. (2018). Characterization of the skin microbiota of the cane toad Rhinella cf. marina in Puerto Rico and Costa Rica. Front. Microbiol. 8, 2624. 10.3389/fmicb.2017.02624 - DOI - PMC - PubMed
-
- Alford, R. A., Brown, G. P., Schwarzkopf, L., Phillips, B. L. and Shine, R. (2009). Comparisons through time and space suggest rapid evolution of dispersal behaviour in an invasive species. Wild Res. 36, 23-28. 10.1071/WR08021 - DOI
-
- Becker, M. H., Richards-Zawacki, C. L., Gratwicke, B. and Belden, L. K. (2014). The effect of captivity on the cutaneous bacterial community of the critically endangered Panamanian golden frog (Atelopus zeteki). Biol. Conserv. 176, 199-206. 10.1016/j.biocon.2014.05.029 - DOI
-
- Belasen, A. M., Riolo, M. A., Bletz, M. C., Lyra, M. L., Toledo, L. F. and James, T. Y. (2021). Geography, host genetics, and cross-domain microbial networks structure the skin microbiota of fragmented Brazilian Atlantic forest frog populations. Ecol. Evol. 11, 9293-9307. 10.1002/ece3.7594 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

