TIM-3 signaling hijacks the canonical Wnt/β-catenin pathway to maintain cancer stemness in acute myeloid leukemia
- PMID: 36745103
- PMCID: PMC10196803
- DOI: 10.1182/bloodadvances.2022008405
TIM-3 signaling hijacks the canonical Wnt/β-catenin pathway to maintain cancer stemness in acute myeloid leukemia
Abstract
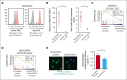
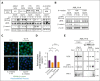
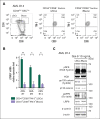
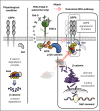
The activation of β-catenin plays critical roles in normal stem cell function, and, when aberrantly activated, the maintenance and enhancement of cancer stemness in many solid cancers. Aberrant β-catenin activation is also observed in acute myeloid leukemia (AML), and crucially contributes to self-renewal and propagation of leukemic stem cells (LSCs) regardless of mutations in contrast with such solid tumors. In this study, we showed that the AML-specific autocrine loop comprised of T-cell immunoglobulin mucin-3 (TIM-3) and its ligand, galectin-9 (Gal-9), drives the canonical Wnt pathway to stimulate self-renewal and propagation of LSCs, independent of Wnt ligands. Gal-9 ligation activates the cytoplasmic Src homology 2 domain of TIM-3 to recruit hematopoietic cell kinase (HCK), a Src family kinase highly expressed in LSCs but not in HSCs, and HCK phosphorylates p120-catenin to promote formation of the LDL receptor-related protein 6 (LRP6) signalosome, hijacking the canonical Wnt pathway. This TIM-3/HCK/p120-catenin axis is principally active in immature LSCs compared with TIM-3-expressed differentiated AML blasts and exhausted T cells. These data suggest that human AML LSCs constitutively activates β-catenin via autocrine TIM-3/HCK/p120-catenin signaling, and that molecules related to this signaling axis should be critical targets for selective eradication of LSCs without impairing normal HSCs.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures




References
-
- Kikushige Y, Miyamoto T, Yuda J, et al. A TIM-3/Gal-9 autocrine stimulatory loop drives self-renewal of human myeloid leukemia stem cells and leukemic progression. Cell Stem Cell. 2015;17(3):341–352. - PubMed
-
- Kikushige Y, Shima T, Takayanagi Si, et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell. 2010;7(6):708–717. - PubMed
-
- Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–541. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

