Effect of Antibiotic Prescription Audit and Feedback on Antibiotic Prescribing in Primary Care: A Randomized Clinical Trial
- PMID: 36745412
- PMCID: PMC9989898
- DOI: 10.1001/jamainternmed.2022.6529
Effect of Antibiotic Prescription Audit and Feedback on Antibiotic Prescribing in Primary Care: A Randomized Clinical Trial
Abstract
Importance: Antibiotics are commonly prescribed in primary care, increasing the risk of antimicrobial resistance in the population.
Objective: To investigate the effect of quarterly audit and feedback on antibiotic prescribing among primary care physicians in Switzerland with medium to high antibiotic prescription rates.
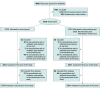
Design, setting, and participants: This pragmatic randomized clinical trial was conducted from January 1, 2018, to December 31, 2019, among 3426 registered primary care physicians and pediatricians in single or small practices in Switzerland who were among the top 75% prescribers of antibiotics. Intention-to-treat analysis was performed using analysis of covariance models and conducted from September 1, 2021, to January 31, 2022.
Interventions: Primary care physicians were randomized in a 1:1 fashion to undergo quarterly antibiotic prescribing audit and feedback with peer benchmarking vs no intervention for 2 years, with 2017 used as the baseline year. Anonymized patient-level claims data from 3 health insurers serving roughly 50% of insurees in Switzerland were used for audit and feedback. The intervention group also received evidence-based guidelines for respiratory tract and urinary tract infection management and community antibiotic resistance information. Physicians in the intervention group were blinded regarding the nature of the trial, and physicians in the control group were not informed of the trial.
Main outcomes and measures: The claims data used for audit and feedback were analyzed to assess outcomes. Primary outcome was the antibiotic prescribing rate per 100 consultations during the second year of the intervention. Secondary end points included overall antibiotic use in the first year and over 2 years, use of quinolones and oral cephalosporins, all-cause hospitalizations, and antibiotic use in 3 age groups.
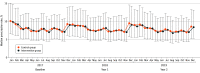
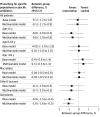
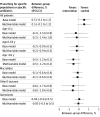
Results: A total of 3426 physicians were randomized to the intervention (n = 1713) and control groups (n = 1713) serving 629 825 and 622 344 patients, respectively, with a total of 4 790 525 consultations in the baseline year of 2017. In the entire cohort, a 4.2% (95% CI, 3.9%-4.6%) relative increase in the antibiotic prescribing rate was noted during the second year of the intervention compared with 2017. In the intervention group, the median annual antibiotic prescribing rate per 100 consultations was 8.2 (IQR, 6.1-11.4) in the second year of the intervention and was 8.4 (IQR, 6.0-11.8) in the control group. Relative to the overall increase, a -0.1% (95% CI, -1.2% to 1.0%) lower antibiotic prescribing rate per 100 consultations was found in the intervention group compared with the control group. No relevant reductions in specific antibiotic prescribing rates were noted between groups except for quinolones in the second year of the intervention (-0.9% [95% CI, -1.5% to -0.4%]).
Conclusions and relevance: This randomized clinical trial found that quarterly personalized antibiotic prescribing audit and feedback with peer benchmarking did not reduce antibiotic prescribing among primary care physicians in Switzerland with medium to high antibiotic prescription rates.
Trial registration: ClinicalTrials.gov Identifier: NCT03379194.
Conflict of interest statement
Figures
Comment in
-
Simplicity Matters-Overengineering Feedback Can Be Counterproductive.JAMA Intern Med. 2023 Mar 1;183(3):220-222. doi: 10.1001/jamainternmed.2022.6528. JAMA Intern Med. 2023. PMID: 36877210 No abstract available.
References
-
- Hawker JI, Smith S, Smith GE, et al. Trends in antibiotic prescribing in primary care for clinical syndromes subject to national recommendations to reduce antibiotic resistance, UK 1995-2011: analysis of a large database of primary care consultations. J Antimicrob Chemother. 2014;69(12):3423-3430. doi: 10.1093/jac/dku291 - DOI - PubMed
-
- Smucny J, Fahey T, Becker L, Glazier R. Antibiotics for acute bronchitis. Cochrane Database Syst Rev. 2004;(4):CD000245. - PubMed

