Prediction of Longitudinal Cognitive Decline in Preclinical Alzheimer Disease Using Plasma Biomarkers
- PMID: 36745413
- PMCID: PMC10087054
- DOI: 10.1001/jamaneurol.2022.5272
Prediction of Longitudinal Cognitive Decline in Preclinical Alzheimer Disease Using Plasma Biomarkers
Abstract
Importance: Alzheimer disease (AD) pathology starts with a prolonged phase of β-amyloid (Aβ) accumulation without symptoms. The duration of this phase differs greatly among individuals. While this disease phase has high relevance for clinical trial designs, it is currently unclear how to best predict the onset of clinical progression.
Objective: To evaluate combinations of different plasma biomarkers for predicting cognitive decline in Aβ-positive cognitively unimpaired (CU) individuals.
Design, setting, and participants: This prospective population-based prognostic study evaluated data from 2 prospective longitudinal cohort studies (the Swedish BioFINDER-1 and the Wisconsin Registry for Alzheimer Prevention [WRAP]), with data collected from February 8, 2010, to October 21, 2020, for the BioFINDER-1 cohort and from August 11, 2011, to June 27, 2021, for the WRAP cohort. Participants were CU individuals recruited from memory clinics who had brain Aβ pathology defined by cerebrospinal fluid (CSF) Aβ42/40 in the BioFINDER-1 study and by Pittsburgh Compound B (PiB) positron emission tomography (PET) in the WRAP study. A total of 564 eligible Aβ-positive and Aβ-negative CU participants with available relevant data from the BioFINDER-1 and WRAP cohorts were included in the study; of those, 171 Aβ-positive participants were included in the main analyses.
Exposures: Baseline P-tau181, P-tau217, P-tau231, glial fibrillary filament protein, and neurofilament light measured in plasma; CSF biomarkers in the BioFINDER-1 cohort, and PiB PET uptake in the WRAP cohort.
Main outcomes and measures: The primary outcome was longitudinal measures of cognition (using the Mini-Mental State Examination [MMSE] and the modified Preclinical Alzheimer Cognitive Composite [mPACC]) over a median of 6 years (range, 2-10 years). The secondary outcome was conversion to AD dementia. Baseline biomarkers were used in linear regression models to predict rates of longitudinal cognitive change (calculated separately). Models were adjusted for age, sex, years of education, apolipoprotein E ε4 allele status, and baseline cognition. Multivariable models were compared based on model R2 coefficients and corrected Akaike information criterion.
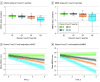
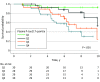
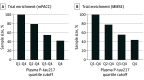
Results: Among 171 Aβ-positive CU participants included in the main analyses, 119 (mean [SD] age, 73.0 [5.4] years; 60.5% female) were from the BioFINDER-1 study, and 52 (mean [SD] age, 64.4 [4.6] years; 65.4% female) were from the WRAP study. In the BioFINDER-1 cohort, plasma P-tau217 was the best marker to predict cognitive decline in the mPACC (model R2 = 0.41) and the MMSE (model R2 = 0.34) and was superior to the covariates-only models (mPACC: R2 = 0.23; MMSE: R2 = 0.04; P < .001 for both comparisons). Results were validated in the WRAP cohort; for example, plasma P-tau217 was associated with mPACC slopes (R2 = 0.13 vs 0.01 in the covariates-only model; P = .01) and MMSE slopes (R2 = 0.29 vs 0.24 in the covariates-only model; P = .046). Sparse models were identified with plasma P-tau217 as a predictor of cognitive decline. Power calculations for enrichment in hypothetical clinical trials revealed large relative reductions in sample sizes when using plasma P-tau217 to enrich for CU individuals likely to experience cognitive decline over time.
Conclusions and relevance: In this study, plasma P-tau217 predicted cognitive decline in patients with preclinical AD. These findings suggest that plasma P-tau217 may be used as a complement to CSF or PET for participant selection in clinical trials of novel disease-modifying treatments.
Conflict of interest statement
Figures
References
-
- Donohue MC, Sperling RA, Petersen R, Sun CK, Weiner MW, Aisen PS; Alzheimer’s Disease Neuroimaging Initiative . Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA. 2017;317(22):2305-2316. doi: 10.1001/jama.2017.6669 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

