Serum Glial Fibrillary Acidic Protein Compared With Neurofilament Light Chain as a Biomarker for Disease Progression in Multiple Sclerosis
- PMID: 36745446
- PMCID: PMC10011932
- DOI: 10.1001/jamaneurol.2022.5250
Serum Glial Fibrillary Acidic Protein Compared With Neurofilament Light Chain as a Biomarker for Disease Progression in Multiple Sclerosis
Abstract
Importance: There is a lack of validated biomarkers for disability progression independent of relapse activity (PIRA) in multiple sclerosis (MS).
Objective: To determine how serum glial fibrillary acidic protein (sGFAP) and serum neurofilament light chain (sNfL) correlate with features of disease progression vs acute focal inflammation in MS and how they can prognosticate disease progression.
Design, setting, and participants: Data were acquired in the longitudinal Swiss MS cohort (SMSC; a consortium of tertiary referral hospitals) from January 1, 2012, to October 20, 2022. The SMSC is a prospective, multicenter study performed in 8 centers in Switzerland. For this nested study, participants had to meet the following inclusion criteria: cohort 1, patients with MS and either stable or worsening disability and similar baseline Expanded Disability Status Scale scores with no relapses during the entire follow-up; and cohort 2, all SMSC study patients who had initiated and continued B-cell-depleting treatment (ie, ocrelizumab or rituximab).
Exposures: Patients received standard immunotherapies or were untreated.
Main outcomes and measures: In cohort 1, sGFAP and sNfL levels were measured longitudinally using Simoa assays. Healthy control samples served as the reference. In cohort 2, sGFAP and sNfL levels were determined cross-sectionally.
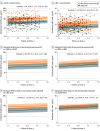
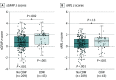
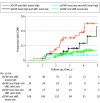
Results: This study included a total of 355 patients (103 [29.0%] in cohort 1: median [IQR] age, 42.1 [33.2-47.6] years; 73 female patients [70.9%]; and 252 [71.0%] in cohort 2: median [IQR] age, 44.3 [33.3-54.7] years; 156 female patients [61.9%]) and 259 healthy controls with a median [IQR] age of 44.3 [36.3-52.3] years and 177 female individuals (68.3%). sGFAP levels in controls increased as a function of age (1.5% per year; P < .001), were inversely correlated with BMI (-1.1% per BMI unit; P = .01), and were 14.9% higher in women than in men (P = .004). In cohort 1, patients with worsening progressive MS showed 50.9% higher sGFAP levels compared with those with stable MS after additional sNfL adjustment, whereas the 25% increase of sNfL disappeared after additional sGFAP adjustment. Higher sGFAP at baseline was associated with accelerated gray matter brain volume loss (per doubling: 0.24% per year; P < .001) but not white matter loss. sGFAP levels remained unchanged during disease exacerbations vs remission phases. In cohort 2, median (IQR) sGFAP z scores were higher in patients developing future confirmed disability worsening compared with those with stable disability (1.94 [0.36-2.23] vs 0.71 [-0.13 to 1.73]; P = .002); this was not significant for sNfL. However, the combined elevation of z scores of both biomarkers resulted in a 4- to 5-fold increased risk of confirmed disability worsening (hazard ratio [HR], 4.09; 95% CI, 2.04-8.18; P < .001) and PIRA (HR, 4.71; 95% CI, 2.05-9.77; P < .001).
Conclusions and relevance: Results of this cohort study suggest that sGFAP is a prognostic biomarker for future PIRA and revealed its complementary potential next to sNfL. sGFAP may serve as a useful biomarker for disease progression in MS in individual patient management and drug development.
Conflict of interest statement
Figures
References
-
- Benkert P, Meier S, Schaedelin S, et al. ; NfL Reference Database in the Swiss Multiple Sclerosis Cohort Study Group . Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21(3):246-257. doi: 10.1016/S1474-4422(22)00009-6 - DOI - PubMed

