Effectiveness of Mycophenolate Mofetil Among Patients With Progressive IgA Nephropathy: A Randomized Clinical Trial
- PMID: 36745456
- PMCID: PMC12578496
- DOI: 10.1001/jamanetworkopen.2022.54054
Effectiveness of Mycophenolate Mofetil Among Patients With Progressive IgA Nephropathy: A Randomized Clinical Trial
Abstract
Importance: The role of mycophenolate mofetil (MMF) in management of immunoglobulin A nephropathy (IgAN) remains highly controversial.
Objective: To evaluate the efficacy and safety of MMF in patients with IgAN at high risk of kidney function loss.
Design, setting, and participants: This randomized clinical trial with open-label, blinded end-point design was conducted among adults with IgAN, proteinuria greater than 1.0 g/d, and estimated glomerular filtration rate (eGFR) greater than 30 and less than 60 mL/min/1.73m2 or with persistent hypertension from September 2013 to December 2015. During a 3-month run-in period, 238 patients received optimized supportive care (SC), including losartan. Patients with a urinary protein excretion rate of 0.75 g/d or greater despite of 3 months optimized SC were enrolled into the trial for 3 years. Survivors of the trial who did not receive dialysis or transplant were followed up after the trial for a median (IQR) of 60 (47-76) months. Data were analyzed from March through June 2022.
Interventions: A total of 170 participants were randomized in a 1:1 ratio to receive MMF (initially, 1.5 g/d for 12 months, maintained at 0.75-1.0 g for at least 6 months) plus SC or SC alone.
Main outcomes and measures: The primary outcomes were (1) a composite of doubling of serum creatinine, end-stage kidney disease (dialysis, transplant, or kidney failure without receiving kidney replacement therapy), or death due to kidney or cardiovascular cause and (2) progression of chronic kidney disease.
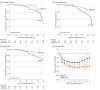
Results: Among 170 randomized patients (mean [SD] age 36.6 [9.4] years; 94 [55.3%] male patients), 85 patients received MMF with SC and 85 patients received SC alone. The mean (SD) eGFR was 50.1 (17.9) mL/min/1.73m2 and mean (SD) proteinuria level was 1.9 (1.7) g/d; 168 patients (98.8%) completed the trial, and 157 participants (92.4%) survived and did not receive dialysis or transplant. Primary composite outcome events occurred in 6 patients (7.1%) in the MMF group and 18 patients (21.2%) in the SC group (adjusted hazard ratio [aHR], 0.23; 95% CI, 0.09-0.63). Progression of chronic kidney disease occurred in 7 participants (8.2%) in the MMF group and 23 participants (27.1%) in the SC group (aHR, 0.23; 95% CI, 0.10-0.57). The effect of MMF treatment on primary outcomes was consistent across prespecified subgroups, with no significant interaction per subgroup. During posttrial follow-up, annual loss of eGFR accelerated after discontinuation of MMF; mean (SD) annual eGFR loss during the study period was 2.9 (1.0) mL/min/1.73m2 in the MMF group and 6.1 (1.2) mL/min/1.73m2 among 66 patients in the MMF group who discontinued MMF after the trial. Serious adverse events were not more frequent with MMF vs SC alone.
Conclusions and relevance: This study found that addition of MMF to SC compared with SC alone significantly reduced risk of disease progression among patients with progressive IgAN.
Trial registration: ClinicalTrials.gov Identifier: NCT01854814.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

