Synchronization of the ovulation and copulation timings increased the number of in vivo fertilized oocytes in superovulated female mice
- PMID: 36745586
- PMCID: PMC9901804
- DOI: 10.1371/journal.pone.0281330
Synchronization of the ovulation and copulation timings increased the number of in vivo fertilized oocytes in superovulated female mice
Abstract
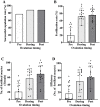
The number of sperm that reaches the oocytes in mammalian species is limited. In mice, 8-10 oocytes are ovulated, a similar number of sperm reaches the oocytes, and nearly all oocytes are fertilized via natural mating. Meanwhile, our improved superovulation technique (ultrasuperovulation: administration of inhibin antiserum and equine chorionic gonadotropin [IASe]) produced 100 oocytes from a single female C57BL/6 mouse but resulted in only approximately 20 fertilized oocytes via mating. We hypothesized that sperm shortage in the ampulla might cause this low fertilization rate. Mice were mated in the proestrus stage or after hormone injection, but ovulation timing was not considered. In clinical application, the rhythm method supports fertilization by testing the ovulation period and synchronizing the ovulation and copulation timings. Therefore, this study examined the effects of ovulation and copulation timings on in vivo fertilization in female mice with IASe. Synchronization of the ovulation and copulation timings increased fertilization efficiency in female mice with ultrasuperovulation. The number of embryos obtained post ovulation was three times higher than that obtained pre ovulation. This study suggests that synchronized ovulation and copulation timings improve the efficiency of in vivo fertilization in IASe-treated female mice. This technique can be used to produce genetically modified mice and develop technologies for infertility treatment.
Copyright: © 2023 Nakao et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

