Denuded Descemet's membrane supports human embryonic stem cell-derived retinal pigment epithelial cell culture
- PMID: 36745611
- PMCID: PMC9901769
- DOI: 10.1371/journal.pone.0281404
Denuded Descemet's membrane supports human embryonic stem cell-derived retinal pigment epithelial cell culture
Abstract
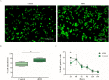
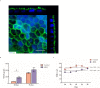
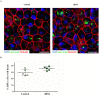
Recent clinical studies suggest that retinal pigment epithelial (RPE) cell replacement therapy may preserve vision in retinal degenerative diseases. Scaffold-based methods are being tested in ongoing clinical trials for delivering pluripotent-derived RPE cells to the back of the eye. The aim of this study was to investigate human embryonic stem cell-derived retinal pigment epithelial (hESC-RPE) cells survival and behaviour on a decellularized Descemet's Membrane (DM), which may be of clinical relevance in retinal transplantation. DMs were isolated from human donor corneas and treated with thermolysin. The DM surface topology and the efficiency of the denudation method were evaluated by atomic force microscope, scanning electron microscopy and histology. hESC-RPE cells were seeded onto the endothelial-side surface of decellularized DM in order to determine the potential of the membrane to support hESC-RPE cell culture, alongside maintaining their viability. Integrity of the hESC-RPE monolayer was assessed by measuring transepithelial resistance. RPE-specific gene expression and growth factors secretion were assessed to confirm maturation and functionality of the cells over the new substrate. Thermolysin treatment did not affect the integrity of the tissue, thus ensuring a reliable method to standardize the preparation of decellularized DM. 24 hours post-seeding, hESC-RPE cell attachment and initial proliferation rate over the denuded DM were higher than hESC-RPE cells cultured on tissue culture inserts. On the new matrix, hESC-RPE cells succeeded in forming an intact monolayer with mature tight junctions. The resulting cell culture showed characteristic RPE cell morphology and proper protein localization. Gene expression analysis and VEGF secretion demonstrate DM provides supportive scaffolding and inductive properties to enhance hESC-RPE cells maturation. Decellularized DM was shown to be capable of sustaining hESC-RPE cells culture, thus confirming to be potentially a suitable candidate for retinal cell therapy.
Copyright: © 2023 Daniele et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2020;9: e144–e160. doi: 10.1016/S2214-109X(20)30489-7 - DOI - PMC - PubMed
-
- Schwartz SD, Pan CK, Klimanskaya I, Lanza R. Chapter 68—Retinal Degeneration. In: Lanza R, Langer R, Vacanti J, editors. Principles of Tissue Engineering (Fourth Edition). Boston: Academic Press; 2014. pp. 1427–1440.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

