Gaucher disease protects against tuberculosis
- PMID: 36745788
- PMCID: PMC7614233
- DOI: 10.1073/pnas.2217673120
Gaucher disease protects against tuberculosis
Abstract
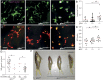
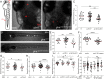
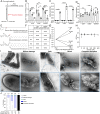
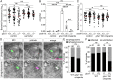
Biallelic mutations in the glucocerebrosidase (GBA1) gene cause Gaucher disease, characterized by lysosomal accumulation of glucosylceramide and glucosylsphingosine in macrophages. Gaucher and other lysosomal diseases occur with high frequency in Ashkenazi Jews. It has been proposed that the underlying mutations confer a selective advantage, in particular conferring protection against tuberculosis. Here, using a zebrafish Gaucher disease model, we find that the mutation GBA1 N370S, predominant among Ashkenazi Jews, increases resistance to tuberculosis through the microbicidal activity of glucosylsphingosine in macrophage lysosomes. Consistent with lysosomal accumulation occurring only in homozygotes, heterozygotes remain susceptible to tuberculosis. Thus, our findings reveal a mechanistic basis for protection against tuberculosis by GBA1 N370S and provide biological plausibility for its selection if the relatively mild deleterious effects in homozygotes were offset by significant protection against tuberculosis, a rampant killer of the young in Europe through the Middle Ages into the 19th century.
Keywords: Gaucher disease; lysosomal glucosylsphingosine; macrophages; tuberculosis resistance; zebrafish.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Cambier C. J., Falkow S., Ramakrishnan L., Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell 159, 1497–1509 (2014). - PubMed
-
- Donovan J., et al. , Adjunctive dexamethasone for the treatment of HIV-uninfected adults with tuberculous meningitis stratified by Leukotriene A4 hydrolase genotype (LAST ACT): Study protocol for a randomised double blind placebo controlled non-inferiority trial. Wellcome Open Res. 3, 32 (2018). - PMC - PubMed
-
- Ramakrishnan L., Mycobacterium tuberculosis pathogenicity viewed through the lens of molecular Koch’s postulates. Curr. Opin. Microbiol. 54, 103–110 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

