Limb girdle muscular disease caused by HMGCR mutation and statin myopathy treatable with mevalonolactone
- PMID: 36745799
- PMCID: PMC9963716
- DOI: 10.1073/pnas.2217831120
Limb girdle muscular disease caused by HMGCR mutation and statin myopathy treatable with mevalonolactone
Abstract
Myopathy is the main adverse effect of the widely prescribed statin drug class. Statins exert their beneficial effect by inhibiting HMG CoA-reductase, the rate-controlling enzyme of the mevalonate pathway. The mechanism of statin myopathy is yet to be resolved, and its treatment is insufficient. Through homozygosity mapping and whole exome sequencing, followed by functional analysis using confocal microscopy and biochemical and biophysical methods, we demonstrate that a distinct form of human limb girdle muscular disease is caused by a pathogenic homozygous loss-of-function missense mutation in HMG CoA reductase (HMGCR), encoding HMG CoA-reductase. We biochemically synthesized and purified mevalonolactone, never administered to human patients before, and establish the safety of its oral administration in mice. We then show that its oral administration is effective in treating a human patient with no significant adverse effects. Furthermore, we demonstrate that oral mevalonolactone resolved statin-induced myopathy in mice. We conclude that HMGCR mutation causes a late-onset severe progressive muscular disease, which shows similar features to statin-induced myopathy. Our findings indicate that mevalonolactone is effective both in the treatment of hereditary HMGCR myopathy and in a murine model of statin myopathy. Further large clinical trials are in place to enable the clinical use of mevalonolactone both in the rare orphan disease and in the more common statin myopathy.
Keywords: HMGCR; limb girdle muscular dystrophy; mutation; statins.
Conflict of interest statement
The authors have patent filings to disclose, A patent has been filed by Y.Y. and O.S.B. Otherwise, the authors have declared that no conflict of interests exists.
Figures


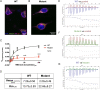

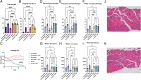
Comment in
-
Rare disease informs mechanism and possible treatment of statin-associated myopathy.Proc Natl Acad Sci U S A. 2023 Mar 7;120(10):e2300988120. doi: 10.1073/pnas.2300988120. Epub 2023 Feb 27. Proc Natl Acad Sci U S A. 2023. PMID: 36848568 Free PMC article. No abstract available.
References
-
- Mercuri E., Bönnemann C. G., Muntoni F., Muscular dystrophies. Lancet 394, 2025–2038 (2019). - PubMed
-
- Pozsgai E., et al. , Unmet needs and evolving treatment for limb girdle muscular dystrophies. Neurodegener. Dis. Manag. 11, 411–429 (2021). - PubMed
-
- Baigent C., et al. , Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366, 1267–78 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

