Novel extragenic genomic safe harbors for precise therapeutic T-cell engineering
- PMID: 36745870
- PMCID: PMC10273162
- DOI: 10.1182/blood.2022018924
Novel extragenic genomic safe harbors for precise therapeutic T-cell engineering
Abstract
Cell therapies that rely on engineered immune cells can be enhanced by achieving uniform and controlled transgene expression in order to maximize T-cell function and achieve predictable patient responses. Although they are effective, current genetic engineering strategies that use γ-retroviral, lentiviral, and transposon-based vectors to integrate transgenes, unavoidably produce variegated transgene expression in addition to posing a risk of insertional mutagenesis. In the setting of chimeric antigen receptor (CAR) therapy, inconsistent and random CAR expression may result in tonic signaling, T-cell exhaustion, and variable T-cell persistence. Here, we report and validate an algorithm for the identification of extragenic genomic safe harbors (GSH) that can be efficiently targeted for DNA integration and can support sustained and predictable CAR expression in human peripheral blood T cells. The algorithm is based on 7 criteria established to minimize genotoxicity by directing transgene integration away from functionally important genomic elements, maximize efficient CRISPR/Cas9-mediated targeting, and avert transgene silencing over time. T cells engineered to express a CD19 CAR at GSH6, which meets all 7 criteria, are curative at low cell dose in a mouse model of acute lymphoblastic leukemia, matching the potency of CAR T cells engineered at the TRAC locus and effectively resisting tumor rechallenge 100 days after their infusion. The identification of functional extragenic GSHs thus expands the human genome available for therapeutic precision engineering.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.O. and M.S. have submitted a patent application partially based on data reported in this manuscript. J.F., J. Eyquem, J.M.-S., and M.S. are named inventors on unrelated patent applications in the field of T-cell engineering. Patent applications are submitted by MSKCC. The remaining authors declare no competing financial interests.
The current affiliation for A.O. is Strand Therapeutics Inc, Boston, MA.
The current affiliation for H.Y. is Calico Life Sciences, San Francisco, CA.
The current affiliation for J.F. is Cluster of Excellence iFIT, University Children's Hospital Tübingen, Tübingen, Germany.
The current affiliation for J. Eyquem is University of Califormia San Francisco, Department of Medicine, Division of Hemato-Oncology, San Francisco, CA.
Figures
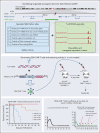






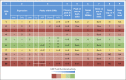
Comment in
-
Splendid isolation.Blood. 2023 Jun 1;141(22):2665-2666. doi: 10.1182/blood.2023019864. Blood. 2023. PMID: 37261853 No abstract available.
References
-
- Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science. 2018;359(6372) - PubMed
-
- Dunbar CE. Stem cell gene transfer: Insights into integration and hematopoiesis from primate genetic marking studies. Ann N Y Acad Sci. 2005;1044:178–182. - PubMed
-
- Bushman F, Lewinski M, Ciuffi A, et al. Genome-wide analysis of retroviral DNA integration. Nat Rev Microbiol. 2005;3(11):848–858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

