Lateral cerebellothalamic tract activation underlies DBS therapy for Essential Tremor
- PMID: 36746367
- PMCID: PMC10200026
- DOI: 10.1016/j.brs.2023.02.002
Lateral cerebellothalamic tract activation underlies DBS therapy for Essential Tremor
Abstract
Background: While deep brain stimulation (DBS) therapy can be effective at suppressing tremor in individuals with medication-refractory Essential Tremor, patient outcome variability remains a significant challenge across centers. Proximity of active electrodes to the cerebellothalamic tract (CTT) is likely important in suppressing tremor, but how tremor control and side effects relate to targeting parcellations within the CTT and other pathways in and around the ventral intermediate (VIM) nucleus of thalamus remain unclear.
Methods: Using ultra-high field (7T) MRI, we developed high-dimensional, subject-specific pathway activation models for 23 directional DBS leads. Modeled pathway activations were compared with post-hoc analysis of clinician-optimized DBS settings, paresthesia thresholds, and dysarthria thresholds. Mixed-effect models were utilized to determine how the six parcellated regions of the CTT and how six other pathways in and around the VIM contributed to tremor suppression and induction of side effects.
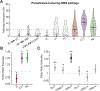
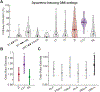
Results: The lateral portion of the CTT had the highest activation at clinical settings (p < 0.05) and a significant effect on tremor suppression (p < 0.001). Activation of the medial lemniscus and posterior-medial CTT was significantly associated with severity of paresthesias (p < 0.001). Activation of the anterior-medial CTT had a significant association with dysarthria (p < 0.05).
Conclusions: This study provides a detailed understanding of the fiber pathways responsible for therapy and side effects of DBS for Essential Tremor, and suggests a model-based programming approach will enable more selective activation of lateral fibers within the CTT.
Keywords: Cerebellothalamic tract; Deep brain stimulation; Essential Tremor; Pathway activation models.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Tara Palnitkar: consultant for Surgical Information Sciences; Remi Patriat: consultant for Surgical Information Sciences; Jerrold Vitek: consultant for Medtronic, Boston Scientific, Abbott, and Surgical Information Sciences; Noam Harel: consultant and a shareholder for Surgical Information Sciences; all other authors declare no conflict of interest.
Figures





References
-
- Siegfried J, Lippitz B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery 1994;35:1126–9. discussion 1129-30. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

