Structural Analogs of the GABAkine KRM-II-81 Are Orally Bioavailable Anticonvulsants without Sedation
- PMID: 36746611
- PMCID: PMC10029819
- DOI: 10.1124/jpet.122.001362
Structural Analogs of the GABAkine KRM-II-81 Are Orally Bioavailable Anticonvulsants without Sedation
Abstract
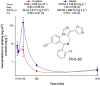
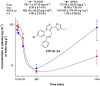
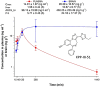
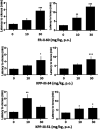
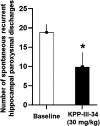
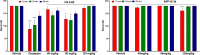
To provide back-up compounds to support the development of the GABAA receptor (GABAAR) potentiator KRM-II-81, three novel analogs were designed: replacing the pyridinyl with 2'-Cl-phenyl (FR-II-60), changing the positions of the N and O atoms in the oxazole ring with addition of an ethyl group (KPP-III-34 and KPP-III-51), or substituting a Br atom for the ethynyl of KRM-II-81 (KPP-III-34). The compounds bound to brain GABAARs. Intraperitoneal administration of FR-II-60 and KPP-III-34 produced anticonvulsant activity in mice [maximal electroshock (MES)-induced seizures or 6 Hz-induced seizures], whereas KPP-III-51 did not. Although all compounds were orally bioavailable, structural changes reduced the plasma and brain (FR-II-60 and KPP-III-51) exposures relative to KRM-II-81. Oral administration of each compound produced dose-dependent increases in the latency for both clonic and tonic seizures and the lethality induced by pentylenetetrazol (PTZ) in mice. Since KPP-III-34 produced the highest brain area under the curve (AUC) exposures, it was selected for further profiling. Oral administration of KPP-III-34 suppressed seizures in corneal-kindled mice, hippocampal paroxysmal discharges in mesial temporal lobe epileptic mice, and PTZ-induced convulsions in rats. Only transient sensorimotor impairment was observed in mice, and doses of KPP-III-34 up to 500 mg/kg did not produce impairment in rats. Molecular docking studies demonstrated that all compounds displayed a reduced propensity for binding to α1His102 compared with the sedating compound alprazolam; the bromine-substituted KPP-III-34 achieved the least interaction. Overall, these findings document the oral bioavailability and anticonvulsant efficacy of three novel analogs of KRM-II-81 with reduced sedative effects. SIGNIFICANCE STATEMENT: A new non-sedating compound, KRM-II-81, with reduced propensity for tolerance is moving into clinical development. Three new analogs were orally bioavailable, produced anticonvulsant effects in rodents, and displayed low sensorimotor impairment. KPP-III-34 demonstrated efficacy in models of pharmacoresistant epilepsy. Docking studies demonstrated a low propensity for compound binding to the α1His102 residue implicated in sedation. Thus, three additional structures have been added to the list of non-sedating imidazodiazepine anticonvulsants that could serve as backups in the clinical development of KRM-II-81.
Copyright © 2023 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
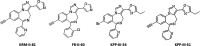
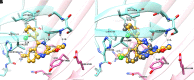
Similar articles
-
The Positive Allosteric Modulator of α2/3-Containing GABAA Receptors, KRM-II-81, Is Active in Pharmaco-Resistant Models of Epilepsy and Reduces Hyperexcitability after Traumatic Brain Injury.J Pharmacol Exp Ther. 2020 Jan;372(1):83-94. doi: 10.1124/jpet.119.260968. Epub 2019 Nov 6. J Pharmacol Exp Ther. 2020. PMID: 31694876 Free PMC article.
-
New Imidazodiazepine Analogue, 5-(8-Bromo-6-(pyridin-2-yl)-4H-benzo[f]imidazo[1,5-a][1,4]diazepin-3-yl)oxazole, Provides a Simplified Synthetic Scheme, High Oral Plasma and Brain Exposures, and Produces Antiseizure Efficacy in Mice, and Antiepileptogenic Activity in Neural Networks in Brain Slices from a Patient with Mesial Temporal Lobe Epilepsy.ACS Chem Neurosci. 2024 Feb 7;15(3):517-526. doi: 10.1021/acschemneuro.3c00555. Epub 2024 Jan 4. ACS Chem Neurosci. 2024. PMID: 38175916
-
Bioisosteres of ethyl 8-ethynyl-6-(pyridin-2-yl)-4H-benzo[f]imidazo [1,5-a][1,4]diazepine-3-carboxylate (HZ-166) as novel alpha 2,3 selective potentiators of GABAA receptors: Improved bioavailability enhances anticonvulsant efficacy.Neuropharmacology. 2018 Jul 15;137:332-343. doi: 10.1016/j.neuropharm.2018.05.006. Epub 2018 May 3. Neuropharmacology. 2018. PMID: 29778948
-
The imidazodiazepine, KRM-II-81: An example of a newly emerging generation of GABAkines for neurological and psychiatric disorders.Pharmacol Biochem Behav. 2022 Feb;213:173321. doi: 10.1016/j.pbb.2021.173321. Epub 2022 Jan 15. Pharmacol Biochem Behav. 2022. PMID: 35041859 Review.
-
The Search for New Screening Models of Pharmacoresistant Epilepsy: Is Induction of Acute Seizures in Epileptic Rodents a Suitable Approach?Neurochem Res. 2017 Jul;42(7):1926-1938. doi: 10.1007/s11064-016-2025-7. Epub 2016 Aug 8. Neurochem Res. 2017. PMID: 27502939 Review.
Cited by
-
ENX-101, a GABAA receptor α2,3,5-selective positive allosteric modulator, displays antiseizure effects in rodent seizure and epilepsy models.Epilepsia. 2025 Jun;66(6):2124-2136. doi: 10.1111/epi.18340. Epub 2025 Mar 15. Epilepsia. 2025. PMID: 40088186 Free PMC article.
-
Identification of New Antiseizure Medication Candidates in Preclinical Animal Studies.Int J Mol Sci. 2023 Aug 24;24(17):13143. doi: 10.3390/ijms241713143. Int J Mol Sci. 2023. PMID: 37685950 Free PMC article. Review.
-
New GABA-Targeting Therapies for the Treatment of Seizures and Epilepsy: II. Treatments in Clinical Development.CNS Drugs. 2023 Sep;37(9):781-795. doi: 10.1007/s40263-023-01025-4. Epub 2023 Aug 21. CNS Drugs. 2023. PMID: 37603261 Free PMC article. Review.
References
-
- Barton ME, Klein BD, Wolf HH, White HS (2001) Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res 47:217–227. - PubMed
-
- Biggerstaff AKivell BSmith JLMian MYGolani LKRashid FSharmin DKnutson DECerne RCook JM, et al. (2020) The α2,3-selective potentiators of GABAA receptors, KRM-II-81 and MP-III-80, produce anxiolytic-like effects and block chemotherapy-induced hyperalgesia in mice without tolerance development. Pharmacol Biochem Behav 196:172996. - PubMed
-
- Bouilleret V, Ridoux V, Depaulis A, Marescaux C, Nehlig A, Le Gal La Salle G (1999) Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy. Neuroscience 89:717–729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

