Targeting Astrocyte Signaling Alleviates Cerebrovascular and Synaptic Function Deficits in a Diet-Based Mouse Model of Small Cerebral Vessel Disease
- PMID: 36746627
- PMCID: PMC10010459
- DOI: 10.1523/JNEUROSCI.1333-22.2023
Targeting Astrocyte Signaling Alleviates Cerebrovascular and Synaptic Function Deficits in a Diet-Based Mouse Model of Small Cerebral Vessel Disease
Abstract
Despite the indispensable role that astrocytes play in the neurovascular unit, few studies have investigated the functional impact of astrocyte signaling in cognitive decline and dementia related to vascular pathology. Diet-mediated induction of hyperhomocysteinemia (HHcy) recapitulates numerous features of vascular contributions to cognitive impairment and dementia (VCID). Here, we used astrocyte targeting approaches to evaluate astrocyte Ca2+ dysregulation and the impact of aberrant astrocyte signaling on cerebrovascular dysfunction and synapse impairment in male and female HHcy diet mice. Two-photon imaging conducted in fully awake mice revealed activity-dependent Ca2+ dysregulation in barrel cortex astrocytes under HHcy. Stimulation of contralateral whiskers elicited larger Ca2+ transients in individual astrocytes of HHcy diet mice compared with control diet mice. However, evoked Ca2+ signaling across astrocyte networks was impaired in HHcy mice. HHcy also was associated with increased activation of the Ca2+/calcineurin-dependent transcription factor NFAT4, which has been linked previously to the reactive astrocyte phenotype and synapse dysfunction in amyloid and brain injury models. Targeting the NFAT inhibitor VIVIT to astrocytes, using adeno-associated virus vectors, led to reduced GFAP promoter activity in HHcy diet mice and improved functional hyperemia in arterioles and capillaries. VIVIT expression in astrocytes also preserved CA1 synaptic function and improved spontaneous alternation performance on the Y maze. Together, the results demonstrate that aberrant astrocyte signaling can impair the major functional properties of the neurovascular unit (i.e., cerebral vessel regulation and synaptic regulation) and may therefore represent a promising drug target for treating VCID and possibly Alzheimer's disease and other related dementias.SIGNIFICANCE STATEMENT The impact of reactive astrocytes in Alzheimer's disease and related dementias is poorly understood. Here, we evaluated Ca2+ responses and signaling in barrel cortex astrocytes of mice fed with a B-vitamin deficient diet that induces hyperhomocysteinemia (HHcy), cerebral vessel disease, and cognitive decline. Multiphoton imaging in awake mice with HHcy revealed augmented Ca2+ responses in individual astrocytes, but impaired signaling across astrocyte networks. Stimulation-evoked arteriole dilation and elevated red blood cell velocity in capillaries were also impaired in cortex of awake HHcy mice. Astrocyte-specific inhibition of the Ca2+-dependent transcription factor, NFAT, normalized cerebrovascular function in HHcy mice, improved synaptic properties in brain slices, and stabilized cognition. Results suggest that astrocytes are a mechanism and possible therapeutic target for vascular-related dementia.
Keywords: Alzheimer's disease; Ca2+; neurovascular coupling; reactive astrocytes; synapses; vascular dementia.
Copyright © 2023 Sompol et al.
Figures
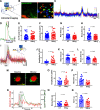
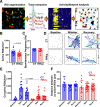
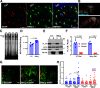
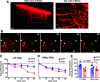

Comment in
-
Targeting Reactive Astrocytes in Vascular Dementia: Investigation of Neuronal-Astrocyte-Vascular Interactions.Neurosci Insights. 2024 May 22;19:26331055241255332. doi: 10.1177/26331055241255332. eCollection 2024. Neurosci Insights. 2024. PMID: 38784154 Free PMC article.
References
-
- Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, LeVine H 3rd, Kraner SD, Norris CM (2009) Cognitive decline in Alzheimer's disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci 29:12957–12969. 10.1523/JNEUROSCI.1064-09.2009 - DOI - PMC - PubMed
-
- Ali M, Falkenhain K, Njiru BN, Murtaza-Ali M, Ruiz-Uribe NE, Haft-Javaherian M, Catchers S, Nishimura N, Schaffer CB, Bracko O (2022) VEGF signalling causes stalls in brain capillaries and reduces cerebral blood flow in Alzheimer's mice. Brain 145:1449–1463. 10.1093/brain/awab387 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
