H-NOX Regulates Biofilm Formation in Agrobacterium Vitis in Response to NO
- PMID: 36746768
- PMCID: PMC10332389
- DOI: 10.1021/acs.biochem.2c00639
H-NOX Regulates Biofilm Formation in Agrobacterium Vitis in Response to NO
Abstract
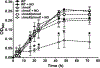
Transitions between motile and biofilm lifestyles are highly regulated and fundamental to microbial pathogenesis. H-NOX (heme-nitric oxide/oxygen-binding domain) is a key regulator of bacterial communal behaviors, such as biofilm formation. A predicted bifunctional cyclic di-GMP metabolizing enzyme, composed of diguanylate cyclase and phosphodiesterase (PDE) domains (avi_3097), is annotated downstream of an hnoX gene in Agrobacterium vitis S4. Here, we demonstrate that avH-NOX is a nitric oxide (NO)-binding hemoprotein that binds to and regulates the activity of avi_3097 (avHaCE; H-NOX-associated cyclic di-GMP processing enzyme). Kinetic analysis of avHaCE indicates a ∼four-fold increase in PDE activity in the presence of NO-bound avH-NOX. Biofilm analysis with crystal violet staining reveals that low concentrations of NO reduce biofilm growth in the wild-type A. vitis S4 strain, but the mutant ΔhnoX strain has no NO phenotype, suggesting that H-NOX is responsible for the NO biofilm phenotype in A. vitis. Together, these data indicate that avH-NOX enhances cyclic di-GMP degradation to reduce biofilm formation in response to NO in A. vitis.
Figures






Similar articles
-
Nitric oxide regulation of cyclic di-GMP synthesis and hydrolysis in Shewanella woodyi.Biochemistry. 2012 Mar 13;51(10):2087-99. doi: 10.1021/bi201753f. Epub 2012 Mar 5. Biochemistry. 2012. PMID: 22360279
-
A structural basis for the regulation of an H-NOX-associated cyclic-di-GMP synthase/phosphodiesterase enzyme by nitric oxide-bound H-NOX.Biochemistry. 2014 Apr 8;53(13):2126-35. doi: 10.1021/bi401597m. Epub 2014 Mar 26. Biochemistry. 2014. PMID: 24628400 Free PMC article.
-
A Pterin-Dependent Signaling Pathway Regulates a Dual-Function Diguanylate Cyclase-Phosphodiesterase Controlling Surface Attachment in Agrobacterium tumefaciens.mBio. 2015 Jun 30;6(4):e00156. doi: 10.1128/mBio.00156-15. mBio. 2015. PMID: 26126849 Free PMC article.
-
Targeting c-di-GMP Signaling, Biofilm Formation, and Bacterial Motility with Small Molecules.Methods Mol Biol. 2017;1657:419-430. doi: 10.1007/978-1-4939-7240-1_31. Methods Mol Biol. 2017. PMID: 28889311 Review.
-
Bacterial diguanylate cyclases: structure, function and mechanism in exopolysaccharide biofilm development.Biotechnol Adv. 2015 Jan-Feb;33(1):124-141. doi: 10.1016/j.biotechadv.2014.11.010. Epub 2014 Dec 10. Biotechnol Adv. 2015. PMID: 25499693 Review.
References
-
- Romling U, and Balsalobre C (2012) Biofilm infections, their resilience to therapy and innovative treatment strategies, J Intern Med 272, 541–561. - PubMed
-
- Stewart PS, and Costerton JW (2001) Antibiotic resistance of bacteria in biofilms, Lancet 358, 135–138. - PubMed
-
- Cary SP, Winger JA, Derbyshire ER, and Marletta MA (2006) Nitric oxide signaling: no longer simply on or off, Trends Biochem Sci 31, 231–239. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

