Regulation of immunological tolerance by the p53-inhibitor iASPP
- PMID: 36746936
- PMCID: PMC9902554
- DOI: 10.1038/s41419-023-05567-9
Regulation of immunological tolerance by the p53-inhibitor iASPP
Abstract
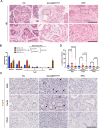
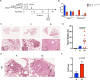
Maintenance of immunological homeostasis between tolerance and autoimmunity is essential for the prevention of human diseases ranging from autoimmune disease to cancer. Accumulating evidence suggests that p53 can mitigate phagocytosis-induced adjuvanticity thereby promoting immunological tolerance following programmed cell death. Here we identify Inhibitor of Apoptosis Stimulating p53 Protein (iASPP), a negative regulator of p53 transcriptional activity, as a regulator of immunological tolerance. iASPP-deficiency promoted lung adenocarcinoma and pancreatic cancer tumorigenesis, while iASPP-deficient mice were less susceptible to autoimmune disease. Immune responses to iASPP-deficient tumors exhibited hallmarks of immunosuppression, including activated regulatory T cells and exhausted CD8+ T cells. Interestingly, iASPP-deficient tumor cells and tumor-infiltrating myeloid cells, CD4+, and γδ T cells expressed elevated levels of PD-1H, a recently identified transcriptional target of p53 that promotes tolerogenic phagocytosis. Identification of an iASPP/p53 axis of immune homeostasis provides a therapeutic opportunity for both autoimmune disease and cancer.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. - PubMed
-
- van Kempen TS, Wenink MH, Leijten EFA, Radstake TRDJ, Boes M. Perception of self: distinguishing autoimmunity from autoinflammation. Nat Rev Rheumatol. 2015;11:483–92. - PubMed
-
- Medzhitov R, Janeway CA. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. - PubMed
-
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

