Small-molecule-mediated OGG1 inhibition attenuates pulmonary inflammation and lung fibrosis in a murine lung fibrosis model
- PMID: 36746968
- PMCID: PMC9902543
- DOI: 10.1038/s41467-023-36314-5
Small-molecule-mediated OGG1 inhibition attenuates pulmonary inflammation and lung fibrosis in a murine lung fibrosis model
Abstract
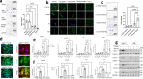
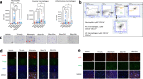
Interstitial lung diseases such as idiopathic pulmonary fibrosis (IPF) are caused by persistent micro-injuries to alveolar epithelial tissues accompanied by aberrant repair processes. IPF is currently treated with pirfenidone and nintedanib, compounds which slow the rate of disease progression but fail to target underlying pathophysiological mechanisms. The DNA repair protein 8-oxoguanine DNA glycosylase-1 (OGG1) has significant roles in the modulation of inflammation and metabolic syndromes. Currently, no pharmaceutical solutions targeting OGG1 have been utilized in the treatment of IPF. In this study we show Ogg1-targeting siRNA mitigates bleomycin-induced pulmonary fibrosis in male mice, highlighting OGG1 as a tractable target in lung fibrosis. The small molecule OGG1 inhibitor, TH5487, decreases myofibroblast transition and associated pro-fibrotic gene expressions in fibroblast cells. In addition, TH5487 decreases levels of pro-inflammatory mediators, inflammatory cell infiltration, and lung remodeling in a murine model of bleomycin-induced pulmonary fibrosis conducted in male C57BL6/J mice. OGG1 and SMAD7 interact to induce fibroblast proliferation and differentiation and display roles in fibrotic murine and IPF patient lung tissue. Taken together, these data suggest that TH5487 is a potentially clinically relevant treatment for IPF but further study in human trials is required.
© 2023. The Author(s).
Conflict of interest statement
T.H. is listed as inventor on a provisional U.S. patent application no. 62/636983, covering OGG1 inhibitors. The patent is fully owned by a nonprofit public foundation, the Helleday Foundation, and T.H. is a member of the foundation board developing OGG1 inhibitors toward the clinic. An inventor reward scheme is under discussion. The remaining authors declare no other competing interests.
Figures






References
-
- Raghu G, et al. Use of a molecular classifier to identify usual interstitial pneumonia in conventional transbronchial lung biopsy samples: a prospective validation study. Lancet Respir. Med. 2019;7:487–496. - PubMed
-
- Martinez FJ, et al. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 2017;3:17074. - PubMed
-
- Kim HJ, Perlman D, Tomic R. Natural history of idiopathic pulmonary fibrosis. Respir. Med. 2015;109:661–670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

