Direct synthesis of ordered mesoporous materials from thermoplastic elastomers
- PMID: 36746971
- PMCID: PMC9902477
- DOI: 10.1038/s41467-023-36362-x
Direct synthesis of ordered mesoporous materials from thermoplastic elastomers
Abstract
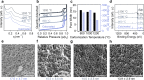
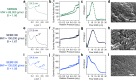
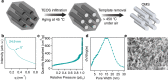
The ability to manufacture ordered mesoporous materials using low-cost precursors and scalable processes is essential for unlocking their enormous potential to enable advancement in nanotechnology. While templating-based methods play a central role in the development of mesoporous materials, several limitations exist in conventional system design, including cost, volatile solvent consumption, and attainable pore sizes from commercial templating agents. This work pioneers a new manufacturing platform for producing ordered mesoporous materials through direct pyrolysis of crosslinked thermoplastic elastomer-based block copolymers. Specifically, olefinic majority phases are selectively crosslinked through sulfonation reactions and subsequently converted to carbon, while the minority block can be decomposed to form ordered mesopores. We demonstrate that this process can be extended to different polymer precursors for synthesizing mesoporous polymer, carbon, and silica. Furthermore, the obtained carbons possess large mesopores, sulfur-doped carbon framework, with tailorable pore textures upon varying the precursor identities.
© 2023. The Author(s).
Conflict of interest statement
Z.Q., M.R. and P.S. submitted a U.S. provisional patent for relevant technology of OMC synthesis using TPE (Serial number: 63/311,804). The remaining authors declare no competing interests.
Figures


References
-
- Liang C, Li Z, Dai S. Mesoporous carbon materials: synthesis and modification. Angew. Chem. Int. Ed. 2008;47:3696–3717. - PubMed
-
- Robertson M, Zagho MM, Nazarenko S, Qiang Z. Mesoporous carbons from self-assembled polymers. J. Polym. Sci. 2022;60:2015–2042.
-
- Eftekhari A, Fan Z. Ordered mesoporous carbon and its applications for electrochemical energy storage and conversion. Mater. Chem. Front. 2017;1:1001–1027.
-
- Qiang Z, et al. Ultra-long cycle life, low-cost room temperature sodium-sulfur batteries enabled by highly doped (N,S) nanoporous carbons. Nano Energy. 2017;32:59–66.
-
- Zhang J, et al. Modification of ordered mesoporous carbon for removal of environmental contaminants from aqueous phase: A review. J. Hazard. Mater. 2021;418:126266. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

