Effects of laboratory domestication on the rodent gut microbiome
- PMID: 36747007
- PMCID: PMC9723573
- DOI: 10.1038/s43705-021-00053-9
Effects of laboratory domestication on the rodent gut microbiome
Abstract
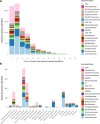
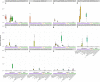
The domestication of the laboratory mouse has influenced the composition of its native gut microbiome, which is now known to differ from that of its wild ancestor. However, limited exploration of the rodent gut microbiome beyond the model species Mus musculus has made it difficult to interpret microbiome variation in a broader phylogenetic context. Here, we analyse 120 de novo and 469 public metagenomically-sequenced faecal and caecal samples from 16 rodent hosts representing wild, laboratory and captive lifestyles. Distinct gut bacterial communities were observed between rodent host genera, with broadly distributed species originating from the as-yet-uncultured bacterial genera UBA9475 and UBA2821 in the families Oscillospiraceae and Lachnospiraceae, respectively. In laboratory mice, Helicobacteraceae were generally depleted relative to wild mice and specific Muribaculaceae populations were enriched in different laboratory facilities, suggesting facility-specific outgrowths of this historically dominant rodent gut family. Several bacterial families of clinical interest, including Akkermansiaceae, Streptococcaceae and Enterobacteriaceae, were inferred to have gained over half of their representative species in mice within the laboratory environment, being undetected in most wild rodents and suggesting an association between laboratory domestication and pathobiont emergence.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Alessandri G, Milani C, Mancabelli L, Mangifesta M, Lugli GA, Viappiani A, et al. The impact of human-facilitated selection on the gut microbiota of domesticated mammals. FEMS Microbiol Ecol. 2019;95. 10.1093/femsec/fiz121 - PubMed
-
- Staats J The Laboratory Mouse. In: Green EL, editor. Biology of the laboratory mouse. New York: Dover Publications; 1966. p. 1-9.

