Impact of population based indoor residual spraying with and without mass drug administration with dihydroartemisinin-piperaquine on malaria prevalence in a high transmission setting: a quasi-experimental controlled before-and-after trial in northeastern Uganda
- PMID: 36747133
- PMCID: PMC9901833
- DOI: 10.1186/s12879-023-07991-w
Impact of population based indoor residual spraying with and without mass drug administration with dihydroartemisinin-piperaquine on malaria prevalence in a high transmission setting: a quasi-experimental controlled before-and-after trial in northeastern Uganda
Abstract
Background: Declines in malaria burden in Uganda have slowed. Modelling predicts that indoor residual spraying (IRS) and mass drug administration (MDA), when co-timed, have synergistic impact. This study investigated additional protective impact of population-based MDA on malaria prevalence, if any, when added to IRS, as compared with IRS alone and with standard of care (SOC).
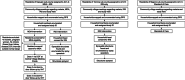
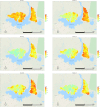
Methods: The 32-month quasi-experimental controlled before-and-after trial enrolled an open cohort of residents (46,765 individuals, 1st enumeration and 52,133, 4th enumeration) of Katakwi District in northeastern Uganda. Consented participants were assigned to three arms based on residential subcounty at study start: MDA+IRS, IRS, SOC. IRS with pirimiphos methyl and MDA with dihydroartemisinin- piperaquine were delivered in 4 co-timed campaign-style rounds 8 months apart. The primary endpoint was population prevalence of malaria, estimated by 6 cross-sectional surveys, starting at baseline and preceding each subsequent round.
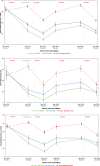
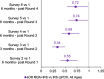
Results: Comparing malaria prevalence in MDA+IRS and IRS only arms over all 6 surveys (intention-to-treat analysis), roughly every 6 months post-interventions, a geostatistical model found a significant additional 15.5% (95% confidence interval (CI): [13.7%, 17.5%], Z = 9.6, p = 5e-20) decrease in the adjusted odds ratio (aOR) due to MDA for all ages, a 13.3% reduction in under 5's (95% CI: [10.5%, 16.8%], Z = 4.02, p = 5e-5), and a 10.1% reduction in children 5-15 (95% CI: [8.5%, 11.8%], Z = 4.7, p = 2e-5). All ages residents of the MDA + IRS arm enjoyed an overall 80.1% reduction (95% CI: [80.0%, 83.0%], p = 0.0001) in odds of qPCR confirmed malaria compared with SOC residents. Secondary difference-in-difference analyses comparing surveys at different timepoints to baseline showed aOR (MDA + IRS vs IRS) of qPCR positivity between 0.28 and 0.66 (p < 0.001). Of three serious adverse events, one (nonfatal) was considered related to study medications. Limitations include the initial non-random assignment of study arms, the single large cluster per arm, and the lack of an MDA-only arm, considered to violate equipoise.
Conclusions: Despite being assessed at long time points 5-7 months post-round, MDA plus IRS provided significant additional protection from malaria infection over IRS alone. Randomized trials of MDA in large areas undergoing IRS recommended as well as cohort studies of impact on incidence.
Trial registration: This trial was retrospectively registered 11/07/2018 with the Pan African Clinical Trials Registry (PACTR201807166695568).
Keywords: Controlled trial; Dihydroartemisinin; High burden; IRS; MDA; Malaria; Pirimiphos; Uganda.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- World Health Organization. High burden to high impact: a targeted malaria response. World Health Organization. 2018.
-
- World Health Organization. World Malaria Report 2019. World Health Organization. 2020.
-
- World Health Organization. World Malaria Report 2020: 20 years of global progress and challenges. World Health Organization. 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

