Safety of metformin continuation in diabetic patients undergoing invasive coronary angiography: the NO-STOP single arm trial
- PMID: 36747244
- PMCID: PMC9902064
- DOI: 10.1186/s12933-023-01744-4
Safety of metformin continuation in diabetic patients undergoing invasive coronary angiography: the NO-STOP single arm trial
Abstract
Background: Despite paucity of data, it is common practice to discontinue metformin before invasive coronary angiography due to an alleged risk of Metformin-Associated Lactic Acidosis (M-ALA). We aimed at assessing the safety of metformin continuation in diabetic patients undergoing coronary angiography in terms of significant increase in lactate levels.
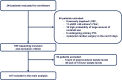
Methods: In this open-label, prospective, multicentre, single-arm trial, all diabetic patients undergoing coronary angiography with or without percutaneous coronary intervention at 3 European centers were screened for enrolment. The primary endpoint was the increase in lactate levels from preprocedural levels at 72-h after the procedure. Secondary endpoints included contrast associated-acute kidney injury (CA-AKI), M-ALA, and all-cause mortality.
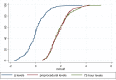
Results: 142 diabetic patients on metformin therapy were included. Median preprocedural lactate level was 1.8 mmol/l [interquartile range (IQR) 1.3-2.3]. Lactate levels at 72 h after coronary angiography were 1.7 mmol/l (IQR 1.3-2.3), with no significant differences as compared to preprocedural levels (p = 0.91; median difference = 0; IQR - 0.5 to 0.4 mmol/l). One patient had 72-h levels ≥ 5 mmol/l (5.3 mmol/l), but no cases of M-ALA were reported. CA-AKI occurred in 9 patients (6.1%) and median serum creatinine and estimated glomerular filtration rate remained similar throughout the periprocedural period. At a median follow-up of 90 days (43-150), no patients required hemodialysis and 2 patients died due to non-cardiac causes.
Conclusions: In diabetic patients undergoing invasive coronary angiography, metformin continuation throughout the periprocedural period does not increase lactate levels and was not associated with any decline in renal function.
Trial registration: The study was registered at Clinicaltrials.gov (NCT04766008).
Keywords: Coronary angiography; Metformin; Metformin-associated lactic acidosis; Percutaneous coronary intervention.
© 2023. The Author(s).
Conflict of interest statement
Dr. Garcia-Garcia reports the following institutional grant support: Biotronik, Boston Scientific, Medtronic, Abbott, Neovasc, Shockwave, Phillips, and Corflow. Dr. Mehran reports grants from Abbott Laboratories, AstraZeneca, Bayer, Beth Israel Deaconess, Bristol Myers Squibb, CSL Behring, DSI, Medtronic, Novartis Pharmaceuticals, OrbusNeich; personal fees from Abbott Laboratories, Boston Scientific, Medscape/WebMD, Siemens Medical Solutions, PLx Opco Inc/dba PLx Pharma Inc, Roivant Sciences, Sanofi, Medtelligence (Janssen Scientific Affairs), Janssen Scientific Affairs; other from Abbott laboratories, other from Abiomed, other from Bristol Myers Squibb, other from Claret Medical, other from Elixir Medical, other from The Medicines eCompany, other from Spectranetics/Philips/Volcano Corp, other from Watermark Research Partners; non-financial support and other from Regeneron Pharmaceuticals, Idorsia Pharmaceuticals Ltd. Dr. Reimers has received speaker honoraria from Boston Scientific. Dr. Stefanini reports a research grant from Boston Scientific and speaker or consulting fees from B. Braun, Biosensors, and Boston Scientific. All other authors report no relationships relevant to the contents of this paper to disclose.
Figures
References
-
- World Diabetes Day. International diabetes federation. 2014. http://www.idf.org/worlddiabetesday/current-campaign.
-
- Colombo A, Godino C, Donahue M, Testa L, Chiarito M, Giulia A, et al. One-year clinical outcome of amphilimus polymer-free drug-eluting stent in diabetes mellitus patients Insight from the ASTUTE registry ( AmphilimuS iTalian mUlticenTre rEgistry ) Int J Cardiol. 2016;214:113–120. doi: 10.1016/j.ijcard.2016.03.088. - DOI - PubMed
-
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

