Rock, scissors, paper: How RNA structure informs function
- PMID: 36747354
- PMCID: PMC10226581
- DOI: 10.1093/plcell/koad026
Rock, scissors, paper: How RNA structure informs function
Abstract
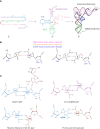
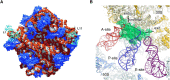
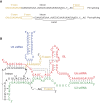
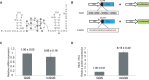
RNA can fold back on itself to adopt a wide range of structures. These range from relatively simple hairpins to intricate 3D folds and can be accompanied by regulatory interactions with both metabolites and macromolecules. The last 50 yr have witnessed elucidation of an astonishing array of RNA structures including transfer RNAs, ribozymes, riboswitches, the ribosome, the spliceosome, and most recently entire RNA structuromes. These advances in RNA structural biology have deepened insight into fundamental biological processes including gene editing, transcription, translation, and structure-based detection and response to temperature and other environmental signals. These discoveries reveal that RNA can be relatively static, like a rock; that it can have catalytic functions of cutting bonds, like scissors; and that it can adopt myriad functional shapes, like paper. We relate these extraordinary discoveries in the biology of RNA structure to the plant way of life. We trace plant-specific discovery of ribozymes and riboswitches, alternative splicing, organellar ribosomes, thermometers, whole-transcriptome structuromes and pan-structuromes, and conclude that plants have a special set of RNA structures that confer unique types of gene regulation. We finish with a consideration of future directions for the RNA structure-function field.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

