This is a preprint.
Scanless two-photon voltage imaging
- PMID: 36747617
- PMCID: PMC9900978
- DOI: 10.21203/rs.3.rs-2412371/v1
Scanless two-photon voltage imaging
Update in
-
Scanless two-photon voltage imaging.Nat Commun. 2024 Jun 14;15(1):5095. doi: 10.1038/s41467-024-49192-2. Nat Commun. 2024. PMID: 38876987 Free PMC article.
Abstract
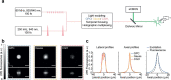
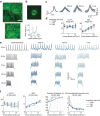
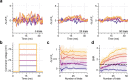
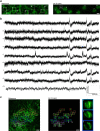
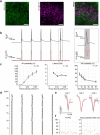
Parallel light-sculpting methods have been used to perform scanless two-photon photostimulation of multiple neurons simultaneously during all-optical neurophysiology experiments. We demonstrate that scanless two-photon excitation also enables high-resolution, high-contrast, voltage imaging by efficiently exciting fluorescence in a large fraction of the cellular soma. We present a thorough characterisation of scanless two-photon voltage imaging using existing parallel approaches and lasers with different repetition rates. We demonstrate voltage recordings of high frequency spike trains and sub-threshold depolarizations in intact brain tissue from neurons expressing the soma-targeted genetically encoded voltage indicator JEDI-2P-kv. Using a low repetition-rate laser, we perform recordings from up to ten neurons simultaneously. Finally, by co-expressing JEDI-2P-kv and the channelrhodopsin ChroME-ST in neurons of hippocampal organotypic slices, we perform single-beam, simultaneous, two-photon voltage imaging and photostimulation. This enables in-situ validation of the precise number and timing of light evoked action potentials and will pave the way for rapid and scalable identification of functional brain connections in intact neural circuits.
Keywords: Two-photon microscopy; computer-generated holography; optogenetics; temporal focusing; voltage imaging.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

