This is a preprint.
Autofluorescence lifetime imaging classifies human lymphocyte activation and subtype
- PMID: 36747690
- PMCID: PMC9900834
- DOI: 10.1101/2023.01.23.525260
Autofluorescence lifetime imaging classifies human lymphocyte activation and subtype
Update in
-
Autofluorescence lifetime imaging classifies human B and NK cell activation state.Front Bioeng Biotechnol. 2025 Apr 4;13:1557021. doi: 10.3389/fbioe.2025.1557021. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40256783 Free PMC article.
Abstract
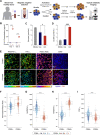
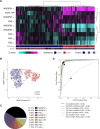
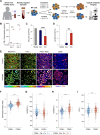
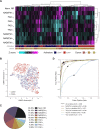
New non-destructive tools are needed to reliably assess lymphocyte function for immune profiling and adoptive cell therapy. Optical metabolic imaging (OMI) is a label-free method that measures the autofluorescence intensity and lifetime of metabolic cofactors NAD(P)H and FAD to quantify metabolism at a single-cell level. Here, we investigate whether OMI can resolve metabolic changes between human quiescent versus IL4/CD40 activated B cells and IL12/IL15/IL18 activated memory-like NK cells. We found that quiescent B and NK cells were more oxidized compared to activated cells. Additionally, the NAD(P)H mean fluorescence lifetime decreased and the fraction of unbound NAD(P)H increased in the activated B and NK cells compared to quiescent cells. Machine learning classified B cells and NK cells according to activation state (CD69+) based on OMI parameters with up to 93.4% and 92.6% accuracy, respectively. Leveraging our previously published OMI data from activated and quiescent T cells, we found that the NAD(P)H mean fluorescence lifetime increased in NK cells compared to T cells, and further increased in B cells compared to NK cells. Random forest models based on OMI classified lymphocytes according to subtype (B, NK, T cell) with 97.8% accuracy, and according to activation state (quiescent or activated) and subtype (B, NK, T cell) with 90.0% accuracy. Our results show that autofluorescence lifetime imaging can accurately assess lymphocyte activation and subtype in a label-free, non-destructive manner.
Keywords: B cells; NK cells; activation; autofluorescence imaging; lymphocytes.
Conflict of interest statement
Competing Interests: RLS, KS, ECG, AJW, and MCS are inventors on patent applications related to this work filed by Wisconsin Alumni Research Foundation (WO2020047133A1, filed on 2019–08-28; US20210049346A1, filed on 2020–08-13; US20210354143A1, filed on 2021–05-17). CMC receives honorarium for advisory board membership with Bayer, Elephas Bio, Nektar Therapeutics, Novartis and WiCell, who had no input in the study design, analysis, manuscript preparation or decision to submit for publication. All other authors declare they have no competing interests.
Figures

References
-
- Omman R. A., Kini A. R., “9 - Leukocyte development, kinetics, and functions” in Rodak’s Hematology (Sixth Edition), Keohane E. M., Otto C. N., Walenga J. M., Eds. (Elsevier, St. Louis (MO), 2020; https://www.sciencedirect.com/science/article/pii/B9780323530453000180), pp. 117–135.
-
- Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S., Functions of natural killer cells. Nat. Immunol. 9, 503–510 (2008). - PubMed
-
- Romee R., Rosario M., Berrien-Elliott M. M., Wagner J. A., Jewell B. A., Schappe T., Leong J. W., Abdel-Latif S., Schneider S. E., Willey S., Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 8, 357ra123–357ra123 (2016). - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
