This is a preprint.
Hacking brain development to test models of sensory coding
- PMID: 36747712
- PMCID: PMC9900841
- DOI: 10.1101/2023.01.25.525425
Hacking brain development to test models of sensory coding
Abstract
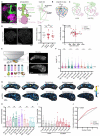
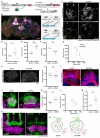
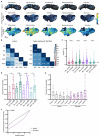
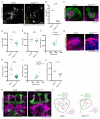
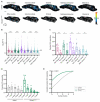
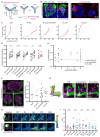
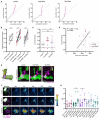
Animals can discriminate myriad sensory stimuli but can also generalize from learned experience. You can probably distinguish the favorite teas of your colleagues while still recognizing that all tea pales in comparison to coffee. Tradeoffs between detection, discrimination, and generalization are inherent at every layer of sensory processing. During development, specific quantitative parameters are wired into perceptual circuits and set the playing field on which plasticity mechanisms play out. A primary goal of systems neuroscience is to understand how material properties of a circuit define the logical operations-computations--that it makes, and what good these computations are for survival. A cardinal method in biology-and the mechanism of evolution--is to change a unit or variable within a system and ask how this affects organismal function. Here, we make use of our knowledge of developmental wiring mechanisms to modify hard-wired circuit parameters in the Drosophila melanogaster mushroom body and assess the functional and behavioral consequences. By altering the number of expansion layer neurons (Kenyon cells) and their dendritic complexity, we find that input number, but not cell number, tunes odor selectivity. Simple odor discrimination performance is maintained when Kenyon cell number is reduced and augmented by Kenyon cell expansion.
Figures
References
-
- Akerboom J., Chen T.-W., Wardill T.J., Tian L., Marvin J.S., Mutlu S., Calderón N.C., Esposti F., Borghuis B.G., Sun X.R., Gordus A., Orger M.B., Portugues R., Engert F., Macklin J.J., Filosa A., Aggarwal A., Kerr R.A., Takagi R., Kracun S., Shigetomi E., Khakh B.S., Baier H., Lagnado L., Wang S.S.-H., Bargmann C.I., Kimmel B.E., Jayaraman V., Svoboda K., Kim D.S., Schreiter E.R., Looger L.L., 2012. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 32, 13819–13840. 10.1523/JNEUROSCI.2601-12.2012 - DOI - PMC - PubMed
-
- Albus J.S., 1971. A theory of cerebellar function. Mathematical Biosciences 10, 25–61. 10.1016/0025-5564(71)90051-4 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
