This is a preprint.
Bioactive multi-protein adsorption enables targeted mast cell nanotherapy
- PMID: 36747749
- PMCID: PMC9901012
- DOI: 10.21203/rs.3.rs-2468299/v1
Bioactive multi-protein adsorption enables targeted mast cell nanotherapy
Update in
-
Controlled adsorption of multiple bioactive proteins enables targeted mast cell nanotherapy.Nat Nanotechnol. 2024 May;19(5):698-704. doi: 10.1038/s41565-023-01584-z. Epub 2024 Jan 16. Nat Nanotechnol. 2024. PMID: 38228804 Free PMC article.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures

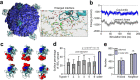

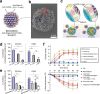
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

