This is a preprint.
Extracellular Matrix Composition Alters Endothelial Force Transmission
- PMID: 36747754
- PMCID: PMC9900979
- DOI: 10.21203/rs.3.rs-2499973/v1
Extracellular Matrix Composition Alters Endothelial Force Transmission
Update in
-
Extracellular matrix composition alters endothelial force transmission.Am J Physiol Cell Physiol. 2023 Jul 1;325(1):C314-C323. doi: 10.1152/ajpcell.00106.2023. Epub 2023 Jun 19. Am J Physiol Cell Physiol. 2023. PMID: 37335028 Free PMC article.
Abstract
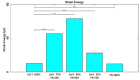
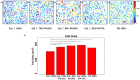
ECM composition is important in a host of pathophysiological processes such as angiogenesis, atherosclerosis, and diabetes, for example and during each of these processes ECM composition has been reported to change over time. However, the impact ECM composition has on the endothelium’s ability to respond mechanically is currently unknown. Therefore, in this study we seeded human umbilical vein endothelial cells (HUVECs) onto soft hydrogels coated with an ECM concentration of 0.1 mg/mL at the following collagen I (Col-I) and fibronectin (FN) ratios: 100%Col-I, 75%Col-I-25%FN, 50%Col-I-50%FN, 25%Col-I-75%FN, and 100%FN. We subsequently measured tractions, intercellular stresses, strain energy, cell morphology, and cell velocity. Our results revealed huvecs seeded on gels coated with 50% Col-I - 50% FN to have the highest intercellular stresses, tractions, strain energies, but the lowest velocities and cell circularity. Huvecs seeded on 100% Col-I had the lowest tractions, cell area while havingthe highest velocities and cell circularity. In addition, cells cultured on 25% Col-I and 75% FN had the lowest intercellular stresses, but the highest cell area. Huvecs cultured on 100% FN yielded the lowest strain energies. We believe these results will be of great importance to the cardiovascular field, biomedical field, and cell mechanics. Summary: Study the influence of different Col-I - FN ECM compositions on endothelial cell mechanics and morphology.
Conflict of interest statement
Conflict of Interest Statement
Ethics approval and consent to participate
Competing interests
The authors declare that they have no competing interests
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

