This is a preprint.
Cannabidiol sensitizes TRPV2 channels to activation by 2-APB
- PMID: 36747846
- PMCID: PMC9900902
- DOI: 10.1101/2023.01.27.525817
Cannabidiol sensitizes TRPV2 channels to activation by 2-APB
Update in
-
Cannabidiol sensitizes TRPV2 channels to activation by 2-APB.Elife. 2023 May 18;12:e86166. doi: 10.7554/eLife.86166. Elife. 2023. PMID: 37199723 Free PMC article.
Abstract
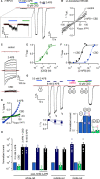
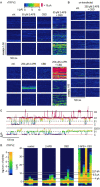
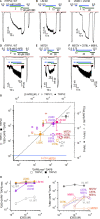
The cation-permeable TRPV2 channel is essential for cardiac and immune cells. Cannabidiol (CBD), a non-psychoactive cannabinoid of clinical relevance, is one of the few molecules known to activate TRPV2. Using the patch-clamp technique we discover that CBD can sensitize current responses of the rat TRPV2 channel to the synthetic agonist 2-aminoethoxydiphenyl borate (2- APB) by over two orders of magnitude, without sensitizing channels to activation by moderate (40 ⁰C) heat. Using cryo-EM we uncover a new small-molecule binding site in the pore domain of rTRPV2 that can be occupied by CBD in addition to a nearby CBD site that had already been reported. The TRPV1 and TRPV3 channels share >40% sequence identity with TRPV2 are also activated by 2-APB and CBD, but we only find a strong sensitizing effect of CBD on the response of mouse TRPV3 to 2-APB. Mutations at non-conserved positions between rTRPV2 and rTRPV1 in either the pore domain or the CBD sites failed to confer strong sensitization by CBD in mutant rTRPV1 channels. Together, our results indicate that CBD-dependent sensitization of TRPV2 channels engages multiple channel regions and possibly involves more than one CBD and 2-APB sites. The remarkably robust effect of CBD on TRPV2 and TRPV3 channels offers a promising new tool to both understand and overcome one of the major roadblocks in the study of these channels - their resilience to activation.
Figures




References
-
- Adams P. D.; Afonine P. V.; Bunkóczi G.; Chen V. B.; Davis I. W.; Echols N.; Headd J. J.; Hung L.-W.; Kapral G. J.; Grosse-Kunstleve R. W.; et al. PHENIX: A Comprehensive Python-Based System for Macromolecular Structure Solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. - PMC - PubMed
-
- Bai D.; del Corsso C.; Srinivas M.; Spray D. C. Block of Specific Gap Junction Channel Subtypes by 2-Aminoethoxydiphenyl Borate (2-APB). J. Pharmacol. Exp. Ther. 2006, 319, 1452–1458. - PubMed
-
- Bang S.; Kim K. Y.; Yoo S.; Lee S.-H.; Hwang S. W. Transient Receptor Potential V2 Expressed in Sensory Neurons Is Activated by Probenecid. Neurosci. Lett. 2007, 425, 120–125. - PubMed
-
- Bisogno T.; Hanus L.; De Petrocellis L.; Tchilibon S.; Ponde D. E.; Brandi I.; Moriello A. S.; Davis J. B.; Mechoulam R.; Di Marzo V. Molecular Targets for Cannabidiol and Its Synthetic Analogues: Effect on Vanilloid VR1 Receptors and on the Cellular Uptake and Enzymatic Hydrolysis of Anandamide. Br. J. Pharmacol. 2001, 134, 845–852. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
