Evaluation of Tcell exhaustion based on the expression of EOMES, Tbet and co-inhibitory receptors in severe and non-severe covid-19 patients
- PMID: 36747893
- PMCID: PMC9892327
- DOI: 10.1016/j.genrep.2023.101747
Evaluation of Tcell exhaustion based on the expression of EOMES, Tbet and co-inhibitory receptors in severe and non-severe covid-19 patients
Abstract
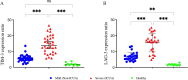
During viral infections, especially Covid-19, Tcell exhaustion plays a crucial role in reducing the activity of lymphocytes and the immune system's antiviral activities. This research aimed to investigate the co-inhibitory receptors and transcription factors involved in the Tcell exhaustion process in ICU-admitted (ICUA) compared to non-ICU admitted (non-ICUA) Covid-19 patients. A total of 60 Covid-19 patients (30 patients in the severe group who were admitted in the ICU (ICUA) and 30 patients in the mild group who were admitted in departments other than the ICU (non-ICUA)) and 10 healthy individuals were included in this study. Laboratory tests and the level of gene expressions related to 4 inhibitory co-receptors, including LAG-3, TIM-3, TIGIT, PD-1, and T-bet and Eomes transcription factors involved in the process of Tcell exhaustion in severe and mild patients of Covid-19 were investigated. The results showed lymphopenia and an increase in other hematologic laboratory factors such as NLR, PLR, CRP, ALT, and AST in people with a severe form of the disease (ICUA) compared to mild groups (non-ICUA) (P < 0.001). Furthermore, a significant increase in 3 co-inhibitory receptors, TIM-3, LAG-3, and PD-1, was observed in severe patients compared to mild and healthy people (P < 0.001). An increase in TIGIT gene expression was lesser than the other three mentioned receptors (P < 0.05). Concerning the transcription factors, we observed a significant increase in Eomes in ICUA patients compared to the non-ICUA group (P < 0.001), and this increment in T-bet gene expression was minor compared to Eomes (P < 0.05). In conclusion, Patients with a severe form of acute respiratory syndrome coronavirus 2 (SARS-CoV-2) represented a higher level of gene expressions in terms of co-inhibitory receptors and transcription factors involved in the T cell exhaustion process.
Keywords: Co-inhibitory receptors; Covid-19; EOMES, eomesodermin; ICU, intensive care units; LAG-3, lymphocyte-activation gene 3; PD-1, programmed cell death protein 1; T-bet, T-box expressed in T; TIGIT, T-cell immunoglobulin and ITIM domain; TIM-3, T cell immunoglobulin and mucin domain-containing protein 3; Tcell exhaustion; Transcription factors.
© 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Angelosanto J.M., Wherry E.J. Transcription factor regulation of CD8+ T-cell memory and exhaustion. Immunol. Rev. 2010;236(1):167–175. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
