Uptake of postplacental intrauterine device placement at cesarean delivery
- PMID: 36748028
- PMCID: PMC9898742
- DOI: 10.1016/j.xagr.2022.100157
Uptake of postplacental intrauterine device placement at cesarean delivery
Abstract
Background: Several studies have investigated the effectiveness of intrauterine device placement at cesarean delivery as a contraceptive method. However, national-level use and outcomes of a postplacental intrauterine device at cesarean delivery are currently understudied in the United States.
Objective: This study aimed to examine the trends, characteristics, and outcomes of patients who received a postplacental intrauterine device at cesarean delivery.
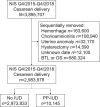
Study design: This retrospective cohort study used the National Inpatient Sample. The study cohort included patients who underwent cesarean delivery from October 2015 to December 2018. The exclusion criteria included hemorrhage, chorioamnionitis, uterine anomaly, hysterectomy, and permanent surgical sterilization. Eligible cases were grouped on the basis of the use of a postplacental intrauterine device at cesarean delivery. The primary outcome measures were temporal trends and characteristics associated with the use of a postplacental intrauterine device at cesarean delivery, assessed using the generalized estimating equation model in multivariable analysis. The secondary outcome measure was perioperative morbidity (leukocytosis, endometritis, myometritis, and sepsis). Propensity score matching was used to balance the baseline characteristics.
Results: Among 2,983,978 patients who met the inclusion criteria, 10,145 patients (0.3%) received a postplacental intrauterine device at cesarean delivery. The use of a postplacental intrauterine device increased from 0.1% in the fourth quarter of 2015 to 0.6% in the fourth quarter of 2018 (P<.001). In a multivariable analysis, the use of a postplacental intrauterine device increased by 14% every quarter-year (adjusted odds ratio, 1.14; 95% confidence interval, 1.13-1.15). In addition, (1) patient characteristics of young age, non-White race, obesity, tobacco use, lowest quartile median household income, and insured with Medicaid; (2) hospital characteristics of large bed capacity and urban teaching setting in Northeast region; and (3) pregnancy characteristics of early gestational age at cesarean delivery, hypertensive disease, previous cesarean delivery, multifetal pregnancy, grand multiparity, placenta previa, and nonelective cesarean delivery represented the independent characteristics associated with the use of a postplacental intrauterine device (all P<.05). A regression tree model identified 35 discrete patterns of the use of a postplacental intrauterine device based on 8 factors (time, race or ethnicity, primary expected payer, obesity, hospital bed capacity, hospital teaching status, hospital region, and previous cesarean delivery). There were 9 patterns, representing 8.8% of the study population, exhibiting a use rate of ≥1.0%, whereas there were 7 patterns, representing 16.0% of the study population, exhibiting no use of a postplacental intrauterine device (absolute rate difference from the highest group to the lowest group, 4.7%). In a propensity score-matched model, postplacental intrauterine device placement at cesarean delivery was not associated with increased risk of measured morbidity (any, 1.8% vs 1.7%; odds ratio, 1.06; 95% confidence interval, 0.66-1.69; P=.812), including postpartum endometritis (1.2% vs 1.0%; odds ratio, 1.19; 95% confidence interval, 0.67-2.14; P=.554).
Conclusion: The use of a postplacental intrauterine device at cesarean delivery increased significantly in recent years in the United States.
Keywords: cesarean delivery; characteristics; morbidity; postplacental intrauterine device; trends.
© 2023 The Authors.
Figures


References
-
- Washington CI, Jamshidi R, Thung SF, Nayeri UA, Caughey AB, Werner EF. Timing of postpartum intrauterine device placement: a cost-effectiveness analysis. Fertil Steril. 2015;103:131–137. - PubMed
-
- Rodriguez MI, Caughey AB, Edelman A, Darney PD, Foster DG. Cost-benefit analysis of state- and hospital-funded postpartum intrauterine contraception at a university hospital for recent immigrants to the United States. Contraception. 2010;81:304–308. - PubMed
-
- Wu M, Eisenberg R, Negassa A, Levi E. Associations between immediate postpartum long-acting reversible contraception and short interpregnancy intervals. Contraception. 2020;102:409–413. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

