Patient-Reported Experiences in Voxelotor-Treated Children and Adults with Sickle Cell Disease: A Semistructured Interview Study
- PMID: 36748060
- PMCID: PMC9899137
- DOI: 10.1155/2023/7533111
Patient-Reported Experiences in Voxelotor-Treated Children and Adults with Sickle Cell Disease: A Semistructured Interview Study
Abstract
Objective: Voxelotor is a first-in-class sickle hemoglobin-polymerization inhibitor that was approved in 2019 by the US Food and Drug Administration for treatment of patients with sickle cell disease (SCD) aged ≥12 years; in 2021, the approval was extended to children with SCD aged 4 to 11 years. Additionally, both the Ministry of Health and Prevention for the United Arab Emirates and the European Commission granted marketing authorization for voxelotor in September 2021 and February 2022, respectively, for treatment of SCD in adults and pediatric patients aged ≥12 years. Thus, additional information on the patient experience with voxelotor would be useful for patients, caregivers, and healthcare professionals alike. The purpose of this study was to conduct semistructured interviews in an effort to understand the experiences and perspectives of voxelotor-treated patients with SCD.
Methods: One-time semistructured interviews with adults, adolescents, and children with SCD and their primary caregivers were conducted in the United States. Twenty-three adults and adolescents were recruited across 4 clinical sites, and 10 children-caregiver dyads were recruited from a single site. The interview was designed to elicit patient perspectives on symptomatic changes with voxelotor and the impact of treatment on patients' perceived health-related quality of life. Individual interview transcripts were analyzed using a thematic analytic approach, and concept saturation was assessed in both cohorts.
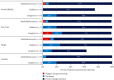
Results: Most patients reported improvements in their SCD symptoms with voxelotor treatment, specifically regarding pain crises, jaundice, and fatigue. Almost all patients experienced improvements in self-reported health-related quality of life with voxelotor treatment.
Conclusions: This study provides patient and caregiver perspectives on the symptomatic benefits of voxelotor treatment. These findings not only highlight the benefits of voxelotor treatment in improving symptoms and increasing health-related quality of life across the entire SCD population but also can inform further research on SCD-specific patient-reported outcomes.
Copyright © 2023 Clark Brown et al.
Conflict of interest statement
Clark Brown is an employee at Global Blood Therapeutics, Inc.; a consultant at Imara and Novo Nordisk; and a recipient of the research funding from Forma Therapeutics, Global Blood Therapeutics, Inc., Imara, and Novartis. Modupe Idowu is on the advisory board at Novartis; on the advisory board and a speaker at Global Blood Therapeutics, Inc.; and a recipient of the research funding from Pfizer Inc., Novartis, Global Blood Therapeutics, Inc., and Forma Therapeutics. Richard Drachtman is a consultant and speaker at Global Blood Therapeutics, Inc. and Agios; a speaker at Jazz Pharmaceuticals; and a consultant at Bluebird Bio. Anne Beaubrun is an employee at Global Blood Therapeutics, Inc. Irene Agodoa and Andy Nguyen are former employees at Global Blood Therapeutics, Inc. Kelly Lipman, Olga Moshkovich, Ryan Murphy, and M. Alex Bellenger are employees at ICON plc. Wally Smith is a consultant at Global Blood Therapeutics, Inc., Novartis, Pfizer Inc., GlycoMimetics, Ironwood, Novo Nordisk, Emmaus Pharmaceuticals, Fera Pharmaceuticals, and Agios Pharmaceuticals and a recipient of the research funding from Global Blood Therapeutics, Inc., NHLBI, PCORI, HRSA, Novartis, Forma Therapeutics, Imara, and Shire.
Figures
Similar articles
-
Modeling the public health impact of voxelotor in the management of sickle cell disease in France.PLoS One. 2023 Sep 13;18(9):e0291211. doi: 10.1371/journal.pone.0291211. eCollection 2023. PLoS One. 2023. PMID: 37703228 Free PMC article.
-
Patient perception of voxelotor treatment benefit in sickle cell disease.J Investig Med. 2022 Jun;70(5):1316-1319. doi: 10.1136/jim-2021-002215. J Investig Med. 2022. PMID: 35732337 Free PMC article.
-
Systematic Review of Voxelotor: A First-in-Class Sickle Hemoglobin Polymerization Inhibitor for Management of Sickle Cell Disease.Pharmacotherapy. 2020 Jun;40(6):525-534. doi: 10.1002/phar.2405. Epub 2020 May 19. Pharmacotherapy. 2020. PMID: 32343424
-
Voxelotor for the treatment of sickle cell disease in pediatric patients.Expert Rev Hematol. 2022 Jun;15(6):485-492. doi: 10.1080/17474086.2022.2082408. Epub 2022 Jun 7. Expert Rev Hematol. 2022. PMID: 35671094 Review.
-
A phase 1/2 ascending dose study and open-label extension study of voxelotor in patients with sickle cell disease.Blood. 2019 Apr 25;133(17):1865-1875. doi: 10.1182/blood-2018-08-868893. Epub 2019 Jan 17. Blood. 2019. PMID: 30655275 Free PMC article. Clinical Trial.
Cited by
-
Hydroxyurea in the sickle cell disease modern era.Expert Rev Clin Pharmacol. 2024 Sep;17(9):777-791. doi: 10.1080/17512433.2024.2390915. Epub 2024 Aug 20. Expert Rev Clin Pharmacol. 2024. PMID: 39135533 Review.
-
Promising role of voxelotor in managing sickle cell disease in children: a narrative review.Clin Exp Pediatr. 2025 Feb;68(2):106-114. doi: 10.3345/cep.2024.00500. Epub 2024 Nov 13. Clin Exp Pediatr. 2025. PMID: 39533724 Free PMC article.
-
Current and Future Therapeutics for Treating Patients with Sickle Cell Disease.Cells. 2024 May 16;13(10):848. doi: 10.3390/cells13100848. Cells. 2024. PMID: 38786070 Free PMC article. Review.
References
-
- Borhade M. B., Kondamudi N. P. Sickle Cell Crisis . Treasure Island (FL): In StatPearls; 2021. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical