Highly Selective Electrocatalytic Reduction of Substituted Nitrobenzenes to Their Aniline Derivatives Using a Polyoxometalate Redox Mediator
- PMID: 36748077
- PMCID: PMC9896480
- DOI: 10.1021/acsorginorgau.2c00047
Highly Selective Electrocatalytic Reduction of Substituted Nitrobenzenes to Their Aniline Derivatives Using a Polyoxometalate Redox Mediator
Abstract
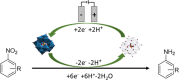
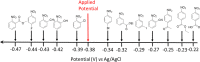
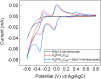
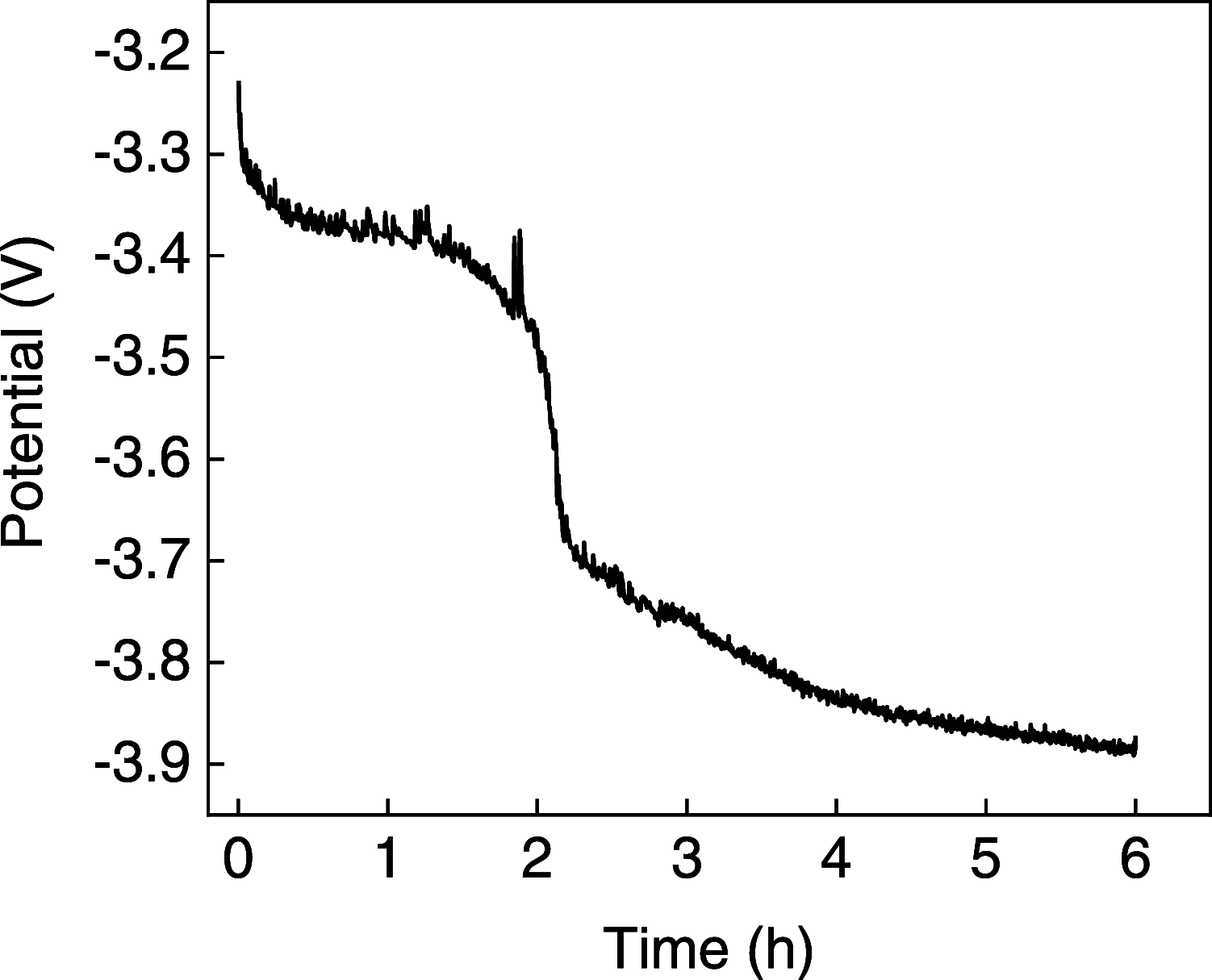
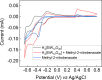
Anilines and substituted anilines are used on the multi-ton scale for producing polymers, pharmaceuticals, dyes, and other important compounds. Typically, these anilines are produced from their corresponding nitrobenzene precursors by reaction with hydrogen at high temperatures. However, this route suffers from a number of drawbacks, including the requirement to handle hydrogen gas, rather harsh reaction conditions that lead to a lack of selectivity and/or toleration of certain functional groups, and questionable environmental sustainability. In light of this, routes to the reduction of nitrobenzenes to their aniline derivatives that operate at room temperature, in aqueous solvent, and without the requirement to use harsh process conditions, hydrogen gas, or sacrificial reagents could be of tremendous benefit. Herein, we report on a highly selective electrocatalytic route for the reduction of nitrobenzenes to their corresponding anilines that works in aqueous solution at room temperature and which does not require the use of hydrogen gas or sacrificial reagents. The method uses a polyoxometalate redox mediator, which reversibly accepts electrons from the cathode and reacts with the nitrobenzenes in solution to reduce them to the corresponding anilines. A variety of substituted nitroarenes are explored as substrates, including those with potentially competing reducible groups and substrates that are difficult to reduce selectively by other means. In all cases, the selectivity for the redox-mediated route is higher than that for the direct reduction of the nitroarene substrates at the electrode, suggesting that redox-mediated electrochemical nitroarene reduction is a promising avenue for the more sustainable synthesis of substituted anilines.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): A patent has been filed relating to the work reported herein.
Figures
References
-
- Vogt P. F.; Gerulis J. J.. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA, 2002.
-
- Kadam H. K.; Tilve S. G. Advancement in methodologies for reduction of nitroarenes. RSC Adv. 2015, 5, 83391–83407. 10.1039/C5RA10076C. - DOI
-
- Ellis F.Paracetamol: A Curriculum Resource; Royal Society of Chemistry: Cambridge, 2002; pp 1–24.
-
- Travis A. S.PATAI’S Chemistry of Functional Groups; John Wiley & Sons: Chichester, UK, 2009.
LinkOut - more resources
Full Text Sources
