Active Immunotherapy for Glioblastoma Treatment: A Systematic Review and Meta-Analysis
- PMID: 36748348
- PMCID: PMC8950026
- DOI: 10.1177/10732748221079474
Active Immunotherapy for Glioblastoma Treatment: A Systematic Review and Meta-Analysis
Abstract
Introduction: Glioblastoma multiforme (GBM) makes 60-70% of gliomas and 15% of primary brain tumors. Despite the availability of standard multimodal therapy, 2 years, 3 years, and 5 years survival rate of GBM are still low. Active immunotherapy is a relatively new treatment option for GBM that seems promising.
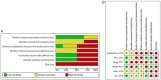
Methods: An electronic database search on PubMed, Cochrane, Scopus, and clinicaltrials.gov was performed to include all relevant studies. This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). Reported parameters are OS, PFS, AEs, post treatment KPS, and 2 year mortality.
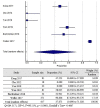
Results: Active immunotherapy provided better OS (HR = .85; 95% CI = .71-1.01; P = .06) and PFS (HS = .83; 95% CI= .66 - 1.03; P = .11) side albeit not statistically significant. Active immunotherapy reduces the risk of 2 year mortality as much as 2.5% compared to control group (NNT and RRR was 56.7078 and 0,0258, respectively).
Conclusion: Active immunotherapy might be beneficial in terms of survival rate in patients with GBM although not statistically significant. It could be a treatment option for GBM in the future.
Keywords: active immunotherapy; glioblastoma; glioblastoma multiforme; high-grade glioma; vaccine.
Conflict of interest statement
Figures
References
-
- Hawasli AH, Kim AH, Dunn GP, Tran DD, Leuthardt EC. Stereotactic laser ablation of high-grade gliomas. Neurosurg Focus. 2014;37:E1. - PubMed
-
- Chen R, Deng X, Wu H, et al. Combined immunotherapy with dendritic cells and cytokine-induced killer cells for malignant tumors: A systematic review and meta-analysis. Int Immunopharm. 2014;22:451-464. - PubMed
-
- Vatu BI, Artene S-A, Staicu A-G, et al. Assessment of efficacy of dendritic cell therapy and viral therapy in high grade glioma clinical trials. A meta-analytic review. J Immunoassay Immunochem. 2019;40:70-80. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical