An ST131 clade and a phylogroup A clade bearing an O101-like O-antigen cluster predominate among bloodstream Escherichia coli isolates from South-West Nigeria hospitals
- PMID: 36748556
- PMCID: PMC9837563
- DOI: 10.1099/mgen.0.000863
An ST131 clade and a phylogroup A clade bearing an O101-like O-antigen cluster predominate among bloodstream Escherichia coli isolates from South-West Nigeria hospitals
Abstract
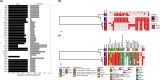
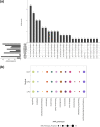
Escherichia coli bloodstream infections are typically attributed to a limited number of lineages that carry virulence factors associated with invasiveness. In Nigeria, the identity of circulating clones is largely unknown and surveillance of their antimicrobial resistance has been limited. We verified and whole-genome sequenced 68 2016-2018 bloodstream E. coli isolates from three sentinel sites in South-Western Nigeria and susceptibility tested 67 of them. Resistance to antimicrobials commonly used in Nigeria was high, with 67 (100 %), 62 (92.5 %), 53 (79.1 %) and 37 (55.2 %) showing resistance to trimethoprim, ampicillin, ciprofloxacin and aminoglycosides, respectively. Thirty-five (51 %) isolates carried extended-spectrum β-lactamase genes and 32 (91 %) of these were multidrug resistant. All the isolates were susceptible to carbapenems and colistin. The strain set included globally disseminated high-risk clones from sequence type (ST)12 (2), ST131 (12) and ST648 (4). Twenty-three (33.8 %) of the isolates clustered within two clades. The first of these consisted of ST131 strains, comprising O16:H5 and O25:H4 sub-lineages. The second was an ST10-ST167 complex clade comprising strains carrying O-antigen and capsular genes of likely Klebsiella origin, identical to those of avian pathogenic E. coli Sanji, and serotyped in silico as O89, O101 or ONovel32, depending on the tool used. Four temporally associated ST90 strains from one sentinel were closely related enough to suggest that at least some of them represented a retrospectively detected outbreak cluster. Our data implicate a broad repertoire of E. coli isolates associated with bloodstream infections in South-West Nigeria. Continued genomic surveillance is valuable for tracking clones of importance and for outbreak identification.
Keywords: Escherichia coli; Nigeria; ST131; antimicrobial resistance; cpsACP; genomic surveillance.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures





References
-
- Sarowska J, Futoma-Koloch B, Jama-Kmiecik A, Frej-Madrzak M, Ksiazczyk M, et al. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: recent reports. Gut Pathog. 2019;11:10. doi: 10.1186/s13099-019-0290-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

