Benefits and challenges in implementation of artificial intelligence in colonoscopy: World Endoscopy Organization position statement
- PMID: 36749036
- PMCID: PMC12136278
- DOI: 10.1111/den.14531
Benefits and challenges in implementation of artificial intelligence in colonoscopy: World Endoscopy Organization position statement
Abstract
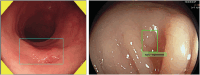
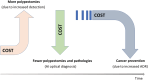
The number of artificial intelligence (AI) tools for colonoscopy on the market is increasing with supporting clinical evidence. Nevertheless, their implementation is not going smoothly for a variety of reasons, including lack of data on clinical benefits and cost-effectiveness, lack of trustworthy guidelines, uncertain indications, and cost for implementation. To address this issue and better guide practitioners, the World Endoscopy Organization (WEO) has provided its perspective about the status of AI in colonoscopy as the position statement. WEO Position Statement: Statement 1.1: Computer-aided detection (CADe) for colorectal polyps is likely to improve colonoscopy effectiveness by reducing adenoma miss rates and thus increase adenoma detection; Statement 1.2: In the short term, use of CADe is likely to increase health-care costs by detecting more adenomas; Statement 1.3: In the long term, the increased cost by CADe could be balanced by savings in costs related to cancer treatment (surgery, chemotherapy, palliative care) due to CADe-related cancer prevention; Statement 1.4: Health-care delivery systems and authorities should evaluate the cost-effectiveness of CADe to support its use in clinical practice; Statement 2.1: Computer-aided diagnosis (CADx) for diminutive polyps (≤5 mm), when it has sufficient accuracy, is expected to reduce health-care costs by reducing polypectomies, pathological examinations, or both; Statement 2.2: Health-care delivery systems and authorities should evaluate the cost-effectiveness of CADx to support its use in clinical practice; Statement 3: We recommend that a broad range of high-quality cost-effectiveness research should be undertaken to understand whether AI implementation benefits populations and societies in different health-care systems.
Keywords: colon polyp; colonoscopy.
© 2023 Japan Gastroenterological Endoscopy Society.
Conflict of interest statement
Y.M.: Olympus Corporation (speaker fee, consultancy and equipment on loan) and Cybernet Systems Corporation (ownership interest). J.E.E.: Satisfai Health (ownership interest and consultancy); Medtronic, Falk and Jannsen (speaker fee); PAION (consultancy). C.H.: Medtronic (consultancy and equipment on loan), Fujifilm (consultancy and equipment on loan) and Pentax (consultancy). O.F.A.: Olympus Corporation (speaker fee). T.M.B.: Medtronic, Wision A.I., Magentiq Eye, DocBot and RSIP Vision (consultancy). M.B.: Satisfai Health (CEO and shareholder). D.v.R.: ERBE, Ventage, Pendopharm, Fujifilm and Pentax (research funding); Boston Scientific, ERBE, Fujifilm and Pendopharm (consultancy). D.G.H.: Olympus Australia Pty Ltd and Fresenius Kabi Pty Ltd (consulting fee); Olympus Australia Pty Ltd, Fresenius Kabi Pty Ltd and Boston Scientific Pty Ltd (speaker honorarium). A.R.: Medtronic and Fujifilm (consultancy and equipment on loan). S.K.: Olympus Corporation (speaker fee) and Cybernet Systems Corporation (ownership interest). M.M.: Olympus Corporation (speaker fee and consultancy), Cybernet Systems Corporation (ownership interest) and Associate Editor of
Figures
References
-
- Mori Y, Kaminski MF, Hassan C, Bretthauer M. Clinical trial designs for artificial intelligence in gastrointestinal endoscopy. Lancet Gastroenterol Hepatol 2022; 7: 785–6. - PubMed
-
- Mori Y, Neumann H, Misawa M, Kudo SE, Bretthauer M. Artificial intelligence in colonoscopy – Now on the market. What's next? J Gastroenterol Hepatol 2021; 36: 7–11. - PubMed
-
- Hsu CC, Sandford BA. The Delphi technique: Making sense of consensus. Pract Assess Res Eval 2007; 12: 10.
-
- Okoli C, Pawlowski SD. The Delphi method as a research tool: An example, design considerations and applications. Inf Manage 2004; 42: 15–29.
-
- Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000; 32: 1008–15. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

