A Randomized Trial of Valganciclovir Prophylaxis Versus Preemptive Therapy in Kidney Transplant Recipients
- PMID: 36749127
- PMCID: PMC10125645
- DOI: 10.1681/ASN.0000000000000090
A Randomized Trial of Valganciclovir Prophylaxis Versus Preemptive Therapy in Kidney Transplant Recipients
Abstract
Significance statement: Although cytomegalovirus (CMV) infection is an important factor in the pathogenesis of kidney allograft rejection, previous studies have not determined the optimal CMV prevention strategy to avoid indirect effects of the virus. In this randomized trial involving 140 kidney transplant recipients, incidence of acute rejection at 12 months was not lower with valganciclovir prophylaxis (for at least 3 months) compared with preemptive therapy initiated after detection of CMV DNA in whole blood. However, prophylaxis was associated with a lower risk of subclinical rejection at 3 months. Although both regimens were effective in preventing CMV disease, the incidence of CMV DNAemia (including episodes with higher viral loads) was significantly higher with preemptive therapy. Further research with long-term follow-up is warranted to better compare the two approaches.
Background: The optimal regimen for preventing cytomegalovirus (CMV) infection in kidney transplant recipients, primarily in reducing indirect CMV effects, has not been defined.
Methods: This open-label, single-center, randomized clinical trial of valganciclovir prophylaxis versus preemptive therapy included kidney transplant recipients recruited between June 2013 and May 2018. After excluding CMV-seronegative recipients with transplants from seronegative donors, we randomized 140 participants 1:1 to receive valganciclovir prophylaxis (900 mg, daily for 3 or 6 months for CMV-seronegative recipients who received a kidney from a CMV-seropositive donor) or preemptive therapy (valganciclovir, 900 mg, twice daily) that was initiated after detection of CMV DNA in whole blood (≥1000 IU/ml) and stopped after two consecutive negative tests (preemptive therapy patients received weekly CMV PCR tests for 4 months). The primary outcome was the incidence of biopsy-confirmed acute rejection at 12 months. Key secondary outcomes included subclinical rejection, CMV disease and DNAemia, and neutropenia.
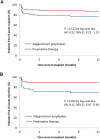
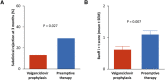
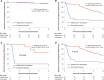
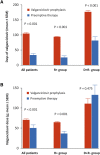
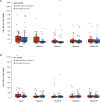
Results: The incidence of acute rejection was lower with valganciclovir prophylaxis than with preemptive therapy (13%, 9/70 versus 23%, 16/70), but the difference was not statistically significant. Subclinical rejection at 3 months was lower in the prophylaxis group (13% versus 29%, P = 0.027). Both regimens prevented CMV disease (in 4% of patients in both groups). Compared with prophylaxis, preemptive therapy resulted in significantly higher rates of CMV DNAemia (44% versus 75%, P < 0.001) and a higher proportion of patients experiencing episodes with higher viral load (≥2000 IU/ml), but significantly lower valganciclovir exposure and neutropenia.
Conclusion: Among kidney transplant recipients, the use of valganciclovir prophylaxis did not result in a significantly lower incidence of acute rejection compared with the use of preemptive therapy.
Clinical trial registry name and registration number: Optimizing Valganciclovir Efficacy in Renal Transplantation (OVERT Study), ACTRN12613000554763 .
Copyright © 2023 by the American Society of Nephrology.
Conflict of interest statement
D. Lysak reports Consultancy: AbbVie, AstraZeneca, Janssen, and Novartis. J. Machová reports Other Interests or Relationships: Member of the Czech Society of Nephrology and Member of the Czech Transplant Society. All remaining authors have nothing to disclose.
Figures


References
-
- Belga S, MacDonald C, Chiang D, Kabbani D, Shojai S, Abraldes JG. Donor graft cytomegalovirus serostatus and the risk of arterial and venous thrombotic events in seronegative recipients after non-thoracic solid organ transplantation. Clin Infect Dis. 2021;72(5):845–852. doi:10.1093/cid/ciaa125 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

