Beyond Breast Density: Risk Measures for Breast Cancer in Multiple Imaging Modalities
- PMID: 36749212
- PMCID: PMC9968778
- DOI: 10.1148/radiol.222575
Beyond Breast Density: Risk Measures for Breast Cancer in Multiple Imaging Modalities
Abstract
Breast density is an independent risk factor for breast cancer. In digital mammography and digital breast tomosynthesis, breast density is assessed visually using the four-category scale developed by the American College of Radiology Breast Imaging Reporting and Data System (5th edition as of November 2022). Epidemiologically based risk models, such as the Tyrer-Cuzick model (version 8), demonstrate superior modeling performance when mammographic density is incorporated. Beyond just density, a separate mammographic measure of breast cancer risk is parenchymal textural complexity. With advancements in radiomics and deep learning, mammographic textural patterns can be assessed quantitatively and incorporated into risk models. Other supplemental screening modalities, such as breast US and MRI, offer independent risk measures complementary to those derived from mammography. Breast US allows the two components of fibroglandular tissue (stromal and glandular) to be visualized separately in a manner that is not possible with mammography. A higher glandular component at screening breast US is associated with higher risk. With MRI, a higher background parenchymal enhancement of the fibroglandular tissue has also emerged as an imaging marker for risk assessment. Imaging markers observed at mammography, US, and MRI are powerful tools in refining breast cancer risk prediction, beyond mammographic density alone.
© RSNA, 2023.
Conflict of interest statement
Figures
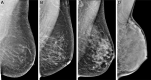
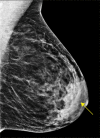
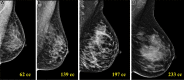
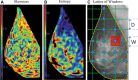
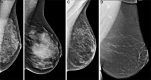
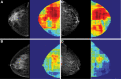
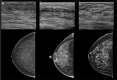

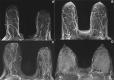
![Spectrum of sonographic and histologic appearance of dense breasts at
mammography. (A) Craniocaudal mammograms show extremely dense fibroglandular
tissue in both cases. (B) Breast US images show predominately hyperechoic
fibrous tissue at one end (left) and abundant isoechoic or hypoechoic
glandular tissue at the other end (right) of the spectrum. (C) Histologic
images (hematoxylin-eosin [H&E] stain; original magnification, x200)
show the breast lobules are involuted and replaced by fibrous stroma in the
former case (left), whereas the lobular involution is minimal and the size
and number of acini per lobule is large in the latter case (right). GTC =
glandular tissue component. (Reprinted, with permission, from reference
70.)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/8de3/9968778/f1463179c75c/radiol.222575.fig9.gif)
References
-
- Johns PC , Yaffe MJ . X-ray characterisation of normal and neoplastic breast tissues . Phys Med Biol 1987. ; 32 ( 6 ): 675 – 695 . - PubMed
-
- Checka CM , Chun JE , Schnabel FR , Lee J , Toth H . The relationship of mammographic density and age: implications for breast cancer screening . AJR Am J Roentgenol 2012. ; 198 ( 3 ): W292 – W295 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

