Selective suppression of de novo SARS-CoV-2 vaccine antibody responses in patients with cancer on B cell-targeted therapy
- PMID: 36749632
- PMCID: PMC10070099
- DOI: 10.1172/jci.insight.163434
Selective suppression of de novo SARS-CoV-2 vaccine antibody responses in patients with cancer on B cell-targeted therapy
Abstract
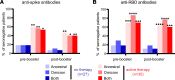
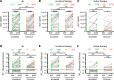
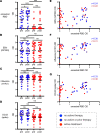
We assessed vaccine-induced antibody responses to the SARS-CoV-2 ancestral virus and Omicron variant before and after booster immunization in 57 patients with B cell malignancies. Over one-third of vaccinated patients at the pre-booster time point were seronegative, and these patients were predominantly on active cancer therapies such as anti-CD20 monoclonal antibody. While booster immunization was able to induce detectable antibodies in a small fraction of seronegative patients, the overall booster benefit was disproportionately evident in patients already seropositive and not receiving active therapy. While ancestral virus- and Omicron variant-reactive antibody levels among individual patients were largely concordant, neutralizing antibodies against Omicron tended to be reduced. Interestingly, in all patients, including those unable to generate detectable antibodies against SARS-CoV-2 spike, we observed comparable levels of EBV- and influenza-reactive antibodies, demonstrating that B cell-targeting therapies primarily impair de novo but not preexisting antibody levels. These findings support rationale for vaccination before cancer treatment.
Keywords: COVID-19; Cancer; Immunoglobulins; Oncology.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

