Circadian regulation of dentate gyrus excitability mediated by G-protein signaling
- PMID: 36749664
- PMCID: PMC10404305
- DOI: 10.1016/j.celrep.2023.112039
Circadian regulation of dentate gyrus excitability mediated by G-protein signaling
Abstract
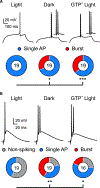
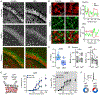
The central circadian regulator within the suprachiasmatic nucleus transmits time of day information by a diurnal spiking rhythm driven by molecular clock genes controlling membrane excitability. Most brain regions, including the hippocampus, harbor similar intrinsic circadian transcriptional machinery, but whether these molecular programs generate oscillations of membrane properties is unclear. Here, we show that intrinsic excitability of mouse dentate granule neurons exhibits a 24-h oscillation that controls spiking probability. Diurnal changes in excitability are mediated by antiphase G-protein regulation of potassium and sodium currents that reduce excitability during the Light phase. Disruption of the circadian transcriptional machinery by conditional deletion of Bmal1 enhances excitability selectively during the Light phase by removing G-protein regulation. These results reveal that circadian transcriptional machinery regulates intrinsic excitability by coordinated regulation of ion channels by G-protein signaling, highlighting a potential novel mechanism of cell-autonomous oscillations.
Keywords: Bmal1; CP: Neuroscience; G-protein; GIRK; NALCN; circadian rhythms; dentate gyrus; excitability; granule cell; intrinsic properties.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

